1
© MathTutorDVD.com
Chemistry 1
Volume 2
Worksheet 5
Mass Percent Composition and Empirical Formulas
Part 2
2
© MathTutorDVD.com
1. Review: What is the empirical formula for the following compounds?
a. C
6
H
6
b. C
2
H
6
O
2
c. CH
4
2. A particular substance has a molar mass of 180 g/mol and an empirical formula of CH
2
O.
What is the molecular formula?
3
© MathTutorDVD.com
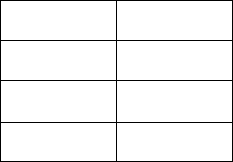
3. A chemist made a new compound and had its elemental composition analyzed. The results
of this analysis are shown below.
Element
Amount
C
1.388 g
H
0.345 g
O
1.850 g
a. What is the percent composition of each of the elements in this compound?
b. What is the empirical formula for this compound?
c. If the molar mass of this compound is 62 g, what is the molecular formula of this
compound?
4
© MathTutorDVD.com
4. A compound with the empirical formula P
2
O
5
was found to have a molar mass of 283.90
g/mol. What is the molecular formula for this compound?
5. A chemist finds a salt on the lab bench and determines it is composed of 64.0 g Br and 9.8 g
of Mg.
a. What is the empirical formula of this compound?
b. Review: What is the name of this compound?
c. Review: What are the charges of the two ions in this compound?
5
© MathTutorDVD.com
6. The percent composition of a sample is as follows: 21.2% N, 24.2% S, 6.1% H, and 48.5% O.
What is the empirical formula of this compound?
7. A compound was determined to have 1.18 g H and 18.8 g O. If the molar mass of the
compound is 34.0 g/mol, find its empirical and molecular formulas.
6
© MathTutorDVD.com
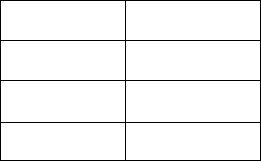
8. A compound was found to contain 0.240 g oxygen and 0.558 g iron.
a. What is the empirical formula of this compound?
b. Review: What is the name of this compound?
9. The elemental composition of a sample is given in the table below.
Element
Amount
C
1.566 g
H
0.392 g
O
1.042 g
a. What is the empirical formula for this compound?
7
© MathTutorDVD.com
b. If the molar mass of this compound is 138.24 g/mol, what is the molecular formula of
this compound?
10. The composition of an ionic compound was determined to be 8.2% nitrogen, 28.3% oxygen,
and 63.5% silver.
a. What is this compound’s empirical formula?
b. Review: What is the name of this compound?

8
© MathTutorDVD.com
Answer Key
1. Review: What is the empirical formula for the following compounds?
a. C
6
H
6
b. C
2
H
6
O
2
c. CH
4
Just divide the subscripts in the formula by the lowest common number. In some cases,
the empirical formula is the same as the molecular formula, as in CH
4
.
Correct answers: a. CH b. CH
3
O c. CH
4
2. A particular substance has a molar mass of 180 g/mol and an empirical formula of CH
2
O.
What is the molecular formula?
Step 1:
Calculate the mass of the empirical formula first.
C H O CH
2
O
Empirical formula mass = 12.01 g/mol + 2(1.01 g/mol) + 15.999 g/mol = 30.029 g/mol.
Step 2:
Divide the molar mass by the empirical formula mass.
!"#
!
"#$
$#%#&'
!
"#$
≈ 6
Step 3:
Use this number and multiply the subscripts in the empirical formula to arrive at the
molecular formula.
6(CH
2
O) = C
6
H
12
O
6
Correct answer: C
6
H
12
O
6
9
© MathTutorDVD.com
3. A chemist made a new compound and had its elemental composition analyzed. The results
of this analysis are shown below.
Element
Amount
C
1.388 g
H
0.345 g
O
1.850
a. What is the percent composition of each of the elements in this compound?
Divide each of the individual masses by the mass of the total sample (3.583 g).
C =
!%$""()
$%*"$()
= 0.387 x 100 = 38.7% C
H =
#%$+*()
$%*"$()
= 0.096 x 100 = 9.6% H
O =
!%"*#()
$%*"$()
= 0.516 x 100 = 51.6% O
Note: Sometimes, the numbers won’t add up to exactly 100% (the sum is 99.9%
in this problem) due to rounding errors, but that’s okay.
Correct answers: 38.7% C; 9.6% H; 51.6% O
b. What is the empirical formula for this compound?
1.388 g C
1 mol C
= 0.116 mol C
12.01 g C
0.345 g H
1 mol H
= 0.342 mol H
1.01 g H
1.850 g O
1 mol O
= 0.116 mol O
15.999 g O

10
© MathTutorDVD.com
Divide each number of moles by the smallest number, 0.116 moles.
C = 0.116 / 0.116 = 1
H = 0.342 / 0.116 = 3
O = 0.116 / 0.116 = 1
This gives the empirical formula: CH
3
O
Correct answer: CH
3
O
c. If the molar mass of this compound is 62 g, what is the molecular formula of this
compound?
Step 1:
Calculate the empirical formula mass first, which is: 31.039 g/mol.
Step 2:
Next, we divide the molecular mass by the empirical mass:
,&()-./0
$!%#$'()-./0
≈ 2
Step 3:
Multiply the subscripts in the empirical formula by this number to get the
molecular formula.
Molecular formula = 2(CH
3
O) = C
2
H
6
O
2
Correct answer: C
2
H
6
O
2
4. A compound with the empirical formula P
2
O
5
was found to have a molecular weight of
283.90 g/mol. What is the molecular formula for this compound?
Molecular weight of the empirical formula = 141.945 g/mol
&"$%'#
!
"#$
!+!%'+*
!
"#$
= 2
Molecular formula = P
4
O
10
Correct answer: P
4
O
10
11
© MathTutorDVD.com
5. A chemist finds a salt on the lab bench and determines it is composed of 64.0 g Br and 9.8 g
of Mg.
a. What is the empirical formula of this compound?
9.8 g Mg
1 mol Mg
= 0.403 mol Mg
24.305 g Mg
64.0 g Br
1 mol Br
= 0.801 mol Br
79.904 g Br
Divide the number of moles of each element by the smallest number of moles.
Mg =
#%+#$(./0
#%+#$(./0
= 1
Br =
#%"#!(./0
#%+#$(./0
= 2
Correct answer: Empirical formula: MgBr
2
b. Review: What is the name of this compound?
Correct answer: Magnesium bromide
c. Review: What are the charges of the two ions in this compound?
Correct answers: Mg
2+
and Br
-
12
© MathTutorDVD.com
6. The percent composition of a sample is as follows: 21.2% N, 24.2% S, 6.1% H, and 48.5% O.
What is the empirical formula of this compound?
To simplify the calculation, we can assume a 100 g sample. This makes it easy to directly
convert the percentages into grams. For example, 21.2 % becomes 21.2 grams when we
assume a 100 g sample.
21.2 g N
1 mol N
= 1.51 mol N
14.01 g N
24.2 g S
1 mol S
= 0.755 mol S
32.065 g S
6.1 g H
1 mol H
= 6.04 mol H
1.01 g H
48.5 g O
1 mol O
= 3.03 mol O
15.999 g O
Now that we have the number of moles of each element, we just divide each number of
moles by the smallest number of moles calculated. In this case, the smallest number is S,
which has 0.755 mol.
N = 1.51 / 0.755 = 2
S = 0.755 / 0.755 = 1
H = 6.04 / 0.755 = 8
O = 3.03 / 0.755 ≈ 4
These ratios become the subscripts of the formula of the compound.
Correct answer: N
2
H
8
SO
4
13
© MathTutorDVD.com
7. A compound was determined to have 1.18 g H and 18.8 g O. If the molar mass of the
compound is 34.0 g/mol, find its empirical and molecular formulas.
1.18 g H
1 mol H
= 1.17 mol H
1.01 g H
18.8 g O
1 mol O
= 1.18 mol O
15.999 g O
Dividing each number of moles by the smallest number of moles to get the empirical
formula, HO.
The molecular mass of HO is 17.009 g/mol.
$+%#
!
"#$
!1%##'
!
"#$
= 2 The molecular formula is H
2
O
2
.
Correct answers: Empirical formula: HO; molecular formula: H
2
O
2
.
8. A compound was found to contain 0.240 g oxygen and 0.558 g iron.
a. What is the empirical formula of this compound?
0.240 g O
1 mol O
= 0.0150 mol O
15.999 g O
0.558 g Fe
1 mol Fe
= 0.0100 mol Fe
55.845 g Fe
O = 0.0150/0.0100 = 1.5
Fe = 0.0100/0.0100 =1
FeO
1.5
Since we need whole numbers, we just multiply the formula by 2 to get Fe
2
O
3
.
Correct answer: Fe
2
O
3
b. Review: What is the name of this compound?
Correct answer: Iron (III) oxide
14
© MathTutorDVD.com
9. The elemental composition of a sample is given in the table below.
Element
Amount
C
1.566 g
H
0.392 g
O
1.42
a. What is the empirical formula for this compound?
1.566 g C
1 mol C
= 0.130 mol C
12.01 g C
0.392 g H
1 mol H
= 0.388 mol H
1.01 g H
1.042 g O
1 mol O
= 0.0651 mol O
15.999 g O
Divide each number of moles by the smallest number to get the empirical
formula: C
2
H
6
O.
Correct answer: C
2
H
6
O
b. If the molar mass of this compound is 138.24 g/mol, what is the molecular formula of
this compound?
!$"%&+
!
"#$
+,%#1'
!
"#$
= 3
Molecular formula = 3(C
2
H
6
O) = C
6
H
18
O
3
Correct answer: C
6
H
18
O
3
15
© MathTutorDVD.com
10. The composition of an ionic compound was determined to be 8.2% nitrogen, 28.3% oxygen,
and 63.5% silver.
a. What is this compound’s empirical formula?
8.2 g N
1 mol N
= 0.585 mol N
14.01 g N
28.3 g O
1 mol O
= 1.77 mol O
15.999 g O
63.5 g Ag
1 mol Ag
= 0.589 mol Ag
107.86 g Ag
Molar ratios: N = 1; O = 3; Ag = 1
Correct answer: Empirical formula: AgNO
3
b. What is the name of this compound?
Correct answer: Silver (I) nitrate
