
1
ADVAMED CODE OF ETHICS ON INTERACTIONS WITH HEALTH CARE PROFESSIONALS
IN INDIA
ADOPTED BY THE ADVAMED BOARD OF DIRECTORS
Effective October 1, 2021
SECTION I – INTRODUCTION
The Advanced Medical Technology Association (AdvaMed) is a global trade association of companies that develop, produce,
manufacture, and market medical devices and products, technologies, digital and software platforms, and related services,
solutions, and therapies used to diagnose, treat, monitor, manage, and alleviate health conditions and disabilities.
The AdvaMed India Executive Committee is an India-based AdvaMed governance group that consists of AdvaMed member
companies’ most senior company executives in India.
The India Executive Committee recognizes the obligation to facilitate ethical interactions between companies, health care
professionals and health care institutions involved in the provision of health care services and/or items to patients in India.
We are dedicated to advancing medical science; developing high quality, innovative medical technology; and improving
patient care.
The Value of Interactions with Health Care Professionals
A health care professional’s first and highest duty is to act in the best interests of their patients. Medical technology
companies help health care professionals meet this duty through necessary, collaborative interactions without interfering with
their professional autonomy and the autonomy of the medical institutions that the health care professionals may be
associated with.
Companies and health care professionals collaborate to advance medical care and clinical science through
research, product development, and product testing that results in new or improved, innovative medical
technology
Companies instruct, educate, and train health care professionals on the safe and effective use of complex
Medical Technology
Companies provide product service and technical support for health care professionals to help ensure the safe
and effective use of Medical Technology
Companies support health care professionals’ scientific and medical research, as well as the enhancement of
clinical skills and educational opportunities to improve patient care
Companies promote charitable giving of medical technologies and public awareness of medical and health
conditions through grants and donations in support of compassionate usage and patient education
2
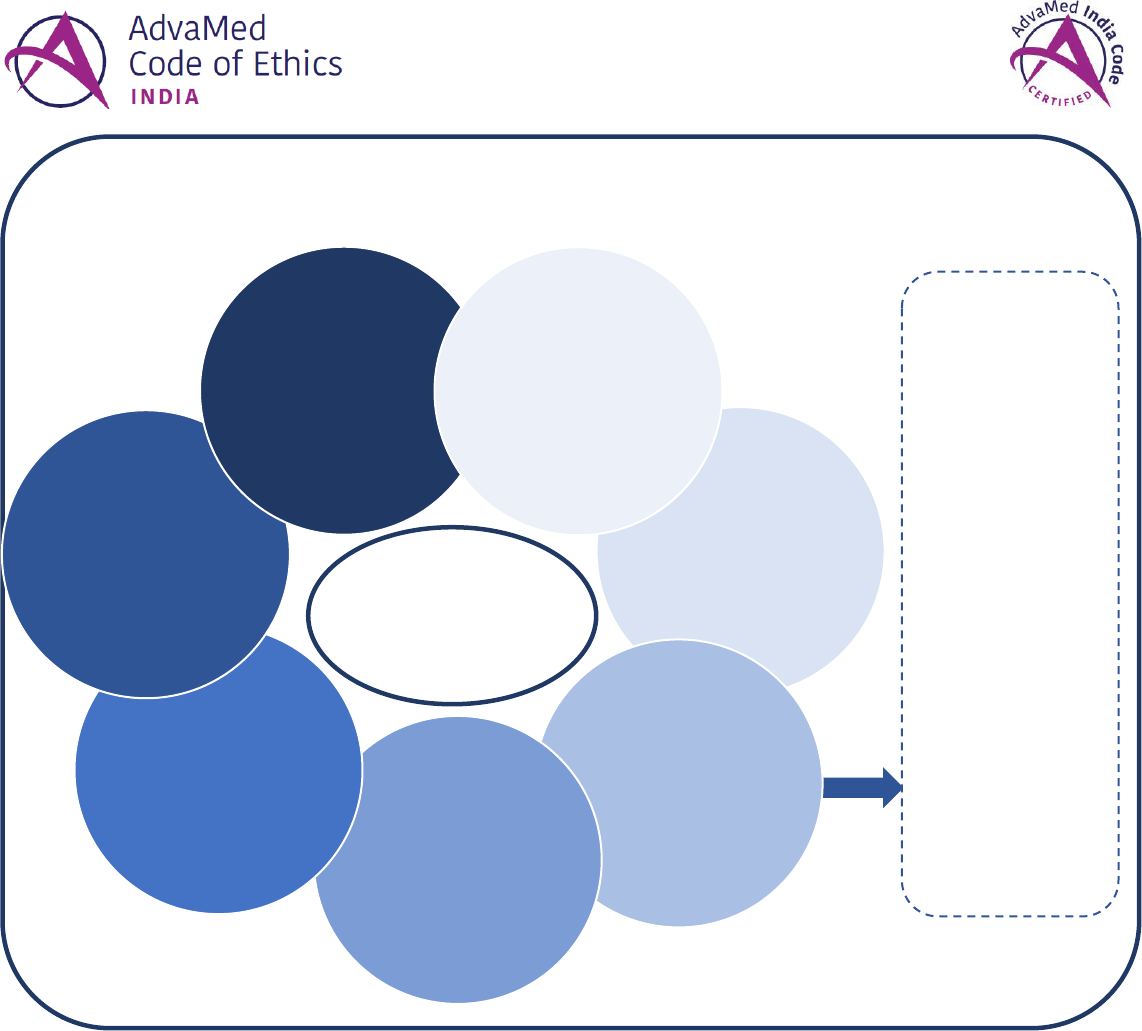
The Purpose of the AdvaMed India Code & Its Cornerstone Values
The AdvaMed India Code (“the Code”) provides medical technology companies with guidance on ethical interactions and
relationships with health care professionals based on the following cornerstone values:
Companies should review all interactions with health care professionals in light of these values and should always avoid
interactions designed to circumvent the Code. The Code may be silent on a specific interaction or may not address all aspects
of an interaction with a health care professional. All interactions between companies and healthcare professionals should
comply with applicable laws, guidelines and codes. The Code is intended to help companies make reasonable and appropriate
decisions that align with the Code’s values.
Companies and their employees, agents and representatives should be mindful of their interactions and the perception of
their interactions with health care professionals. Companies should communicate company policies consistent with the Code
to their employees, agents and representatives with the expectation that they will comply.
Scope and Applicability of the Code
Legal Principles
The Code does not provide legal advice or create legal rights or obligations.
Geographic
Reach
The Code applies to all company interactions with India health care professionals, whether occurring
inside or outside India (such as at a conference or other event).
INNOVATION
EDUCATION
INTEGRITY
RESPECT
RESPONSIBILITY
TRANSPARENCY
Advance the development and availability of safe and effective medical
technology that health care professionals use to improve and save lives
Deliver high-quality training and education to help ensure that health
care professionals safely and effectively use medical technology
Conduct business with integrity at all times and avoid real or perceived
conflicts of interest with health care professionals
Respect the independent clinical judgment of health care professionals
to decide the best manner and method for treating patients
Promote socially and ethically responsible business practices that
protect patients, their rights and their safety
Conduct interactions with health care professionals fairly, openly and
transparently

3
Interactions
with Health Care
Professionals
The Code applies to a company’s interactions and a company’s employees’, representatives’, and
agents’ interactions with India health care professionals, even if an employee or representative or agent
pays for the interaction’s associated costs himself/herself.
Representatives
A company adopting the Code is required to communicate the Code’s provisions to its employees,
agents, representatives, dealers, distributors, vendors, independent consultants acting on a company’s
behalf and to any other health care professionals engaged by or affiliated with a company with the
expectation that they will adhere to the Code.
Multiple
Business Lines
Companies with different business lines (for example, medical devices, pharmaceuticals, biologics,
consumer items, and/or research-only products) may have other industry codes that apply to such
different businesses. This Code applies to companies’ interactions with the healthcare professionals
linked to medical technology.
Combination
Products
The Code also applies to all interactions with India health care professionals related to combination
products that include a medical technology component (for example, those that are both biologics and
devices or drugs and devices), which may also be subject to other trade association codes.
Complying with the AdvaMed India Code
The Code does not replace any applicable laws, regulations, or codes as notified from time to time that may contain stricter
requirements. The Code requires companies to comply with all applicable laws, regulations, and codes. Companies are strongly
encouraged to adopt an effective ethics and compliance program aimed at (1) promoting an organizational culture that
encourages ethical practices and a commitment to comply with the applicable law, guidelines and codes and (2) preventing
and detecting inappropriate conduct. Programs should be appropriately tailored for each company.
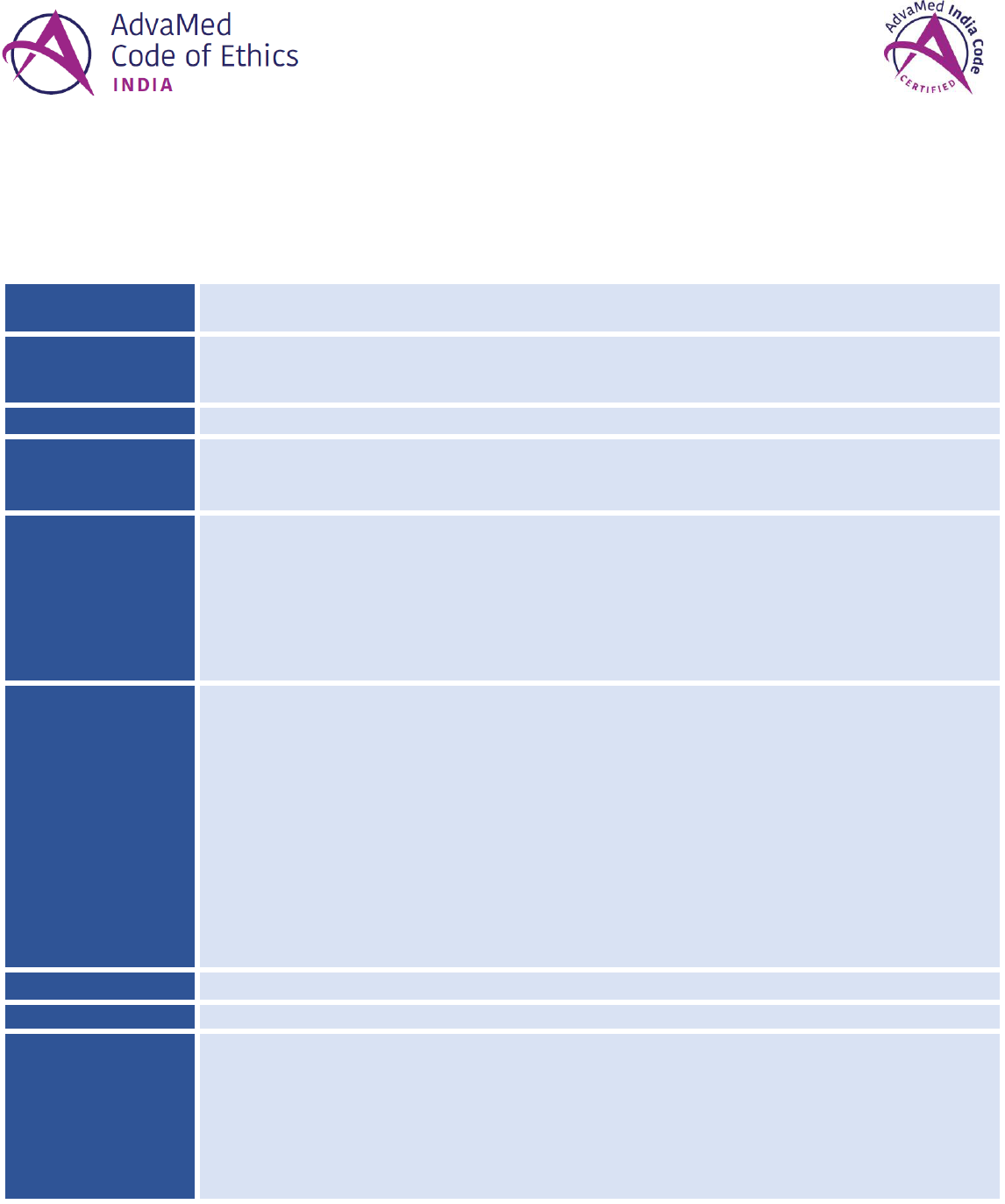
For assistance in evaluating a compliance program’s effectiveness, companies may consider referring to government-issued or
other industry guidance on what constitutes an effective compliance program. Elements of an effective compliance program
can include:
4
All medical technology companies doing business in India are strongly encouraged to adopt and certify to this Code and to
implement an effective compliance program.
A company that wishes to certify to the Code is required to submit to AdvaMed an annual certification that the company has
adopted the Code and has implemented a compliance program designed to uphold the principles of this Code. This
certification must be signed by the most senior executive responsible for the company’s medical technology operation in
India. For companies headquartered in India, this would be the Chief Executive Officer or individual with equivalent
responsibility within the certifying company. For companies headquartered outside of India, this would be the most senior
representative of the certifying company’s medical technology operation in India. This certification must additionally be signed
by the company’s Chief Compliance Officer for India or individual with equivalent responsibilities within the certifying
company.
COMMITMENT
TO ETHICAL
CULTURE
✓ Board & senior
management are
knowledgeable
about and oversees
the compliance
program
✓ Individuals in
leadership with
overall
responsibility for
the compliance
program
✓ Compliance
personnel with day-
to-day program
responsibility,
including
appropriate Board
access and
reporting
✓ Retention of
personnel who
have not engaged
in conduct
inconsistent with
an effective
compliance
program
ELEMENTS OF AN EFFECTIVE COMPLIANCE PROGRAM
Effective training,
education and
communication
Appropriate
oversight and
management of
the compliance
program
Standards
enforced through
disciplinary action
Prompt
response to
detected problems
and corrective
action undertaken
Written policies
& procedures that
incorporate and
foster compliance
with the Code
Internal risk
assessments,
monitoring, and
auditing
Effective lines of
communication
(including an
anonymous
reporting
hotline)
5
AdvaMed will publish on its website a list of those companies that have submitted this annual certification. As part of this
certification, companies must supply contact information for the company’s compliance program or an anonymous hotline to
facilitate reporting of possible violations of the Code. AdvaMed will publish on its website the contact information supplied by
each company.
Glossary
Advertisements
Any activity undertaken, organized or sponsored by a company which is directed at health care
professionals to promote the safe and effective use of medical technology.
Commercial
Sponsorship
A payment or in-kind support provided to a third party in exchange for advertising or promotional
opportunities for a company (for example, a company exhibit at a third-party program).
Company
A company that develops, produces, manufactures and/or markets medical technology.
Educational Grant
A payment or in-kind support to a third-party entity (for example, a third-party program organizer,
training institution or health care organization) to reduce the costs of providing education. An
educational grant is not offered for commercial sponsorship opportunities.
Health Care
Professional
A health care professional is any person or entity (a) authorized or licensed in India to provide
health care services or items to patients or (b) who is involved in the decision to purchase,
prescribe, order, or recommend a medical technology in India. This term includes individual
clinicians (for example, physicians, nurses, and pharmacists, among others), provider entities (for
example, hospitals and ambulatory surgical centers), and administrative personnel at provider
entities in India (for example, hospital purchasing agents). This term does not include health care
professionals who are bona fide employees of a company, while acting in that capacity.
Medical Technology
Medical technology is a broad term that means medical devices and products, technologies, digital
and software platforms, and related services, solutions, and therapies used to diagnose, treat,
monitor, manage, and alleviate health conditions and disabilities. Some examples include:
• Implantable medical devices that are placed in or on the human body to replace, repair, or
strengthen a body part;
• Surgical devices used to perform procedures;
• Digital technology and software platforms that assist in monitoring, diagnosing, and treating
patients; and
• Non-invasive reagents, instrumentation, and/or software to aid in the diagnosis and treatment
of patients; among other technology.
Modest
Moderate value; may differ depending on the local standards and the purpose.
Occasional
An interaction is considered occasional if it occurs infrequently and not on a routine basis.
Satellite Symposium
A satellite symposium is a company-organized and funded program that is appended to a third-party
program agenda but that the third-party organizer does not control. These programs often take
place during meal breaks at the third-party program and may address education and training topics
that coincide with the third-party program’s focus.
A satellite symposium does not include a company-organized meeting, training, or educational
session (such as an advisory board, consultant meeting, or product education session) that (a) may
6
be held in close physical and temporal proximity to a third-party program and (b) is not appended to
or included in the third-party program’s official agenda.
Third-Party Program
A bona fide, independent health care-related educational, scientific, business, and/or policymaking
conference, meeting, or event put on by a third party other than a company. This term includes
programs that are accredited to provide continuing education credits and programs that are not
accredited.
Third-Party Program
Organizer
A third-party entity that organizes and/or oversees the development of the third-party program,
including the selection of presenters, attendees, topics, materials, and methods. A third-party
program organizer could include, for example, a health care professional society, institution,
association, medical trust fund, continuing medical education provider, hospital or other health care
entity.
Consigned Products
Medical technologies (a) that a company provides to a health care professional for use in and
storage at the health care professional’s patient care setting and (b) to which the company retains
title until the product is used.
Demonstration &
Evaluation Products
Products provided to health care professionals at no charge to assess the appropriate use and
functionality of the product and determine whether and when to use, order, purchase, or
recommend the product in the future. Company products that may be provided to health care
professionals for such assessment include single use (for example, samples of consumable or
disposable products) and multiple use products (sometimes referred to as capital equipment).

7
SECTION II – CONSULTING ARRANGEMENTS WITH HEALTH CARE PROFESSIONALS
A. Engaging a Health Care Professional to Provide Consulting Services
Companies engage health care professionals to provide a wide range of valuable, bona fide consulting services. Some
examples include arrangements for a health care professional to provide education and training, speaking services,
proctorships (evaluate), preceptorships (instruct), reference center or center of excellence services, participation on advisory
boards or focus groups, medical technology development and research services arrangements (such as research and
development, clinical studies, clinical investigator services, collaborative research, and post-market research), and
arrangements for the development or transfer of intellectual property.
Companies should apply the following principles to all consulting arrangements with health care professionals:
• Legitimate Need. Companies should enter a consulting arrangement with a health care professional only if the
company has identified a legitimate need for the health care professional’s services in advance.
• Consultant Selection. Companies should select only duly vetted health care professionals to serve as consultants,
based on the health care professional’s qualifications to meet the identified need. Some examples of these
qualifications include the health care professional’s specialty, years of experience, location, practice setting, clinical
research experience, podium presence, speaking and publication experience, or experience with, usage of, or
familiarity with a specific medical technology, among other qualifications. Companies may not select or compensate
consultants as a reward for past usage or as an unlawful inducement for future purchases. Companies should
implement safeguards so that consultants are not selected based in whole or in part on sales considerations.
• Number of Consultants. Companies should engage only as many consultants as are necessary to fulfill the
requirements for the bona fide services.
• Fair Market Value Compensation. Companies should compensate consultants consistent with the fair market value in
an arm’s length transaction of the services provided. Companies should not base compensation on the volume or
value of the consultant’s business generated by the consultant in the past, present or expected in the future.
Companies should confirm that the services performed by the consultant are in accordance with the agreement.
• Expenses. Companies may pay for documented, reasonable, and actual expenses incurred by a consultant that are
necessary to carry out the consulting arrangement, such as costs for travel, lodging, and modest meals. See Sections VI
and VII of the Code for information on providing travel, lodging, and meals to health care professionals.
• Written Agreement. Companies should enter into written agreements that describe all consulting services to be
provided and the compensation to be paid in exchange for the services. Such agreements with the healthcare
professionals should be fully transparent and disclosed (when required to do so by the appropriate authority), should
ensure that the professional integrity and freedom of the health care professionals are maintained and that the patients’
interest is not compromised. When a company contracts with a consultant to conduct clinical research services, there
should also be a written research protocol.
• Sales Involvement. Sales personnel cannot control or unduly influence the decision to engage a particular health care
professional as a consultant. Companies’ sales personnel may provide input about the qualifications of a proposed
consultant. Companies should consider implementing appropriate controls to promote compliance with this section.

8
B. Royalties
Arrangements involving the payment of royalties to health care professionals should meet the standards listed in this section
of the Code.
Health care professionals often make valuable contributions that improve products or medical technologies. They may
develop intellectual property (for example, patents, trade secrets, or know-how), under a product or technology development
or intellectual property licensing agreement.
Companies should enter a royalty arrangement with a health care professional only if the health care professional (individually
or as part of a group) makes a novel, significant, or innovative contribution to the development of a medical technology or a
combination product, process, or method, subject to intellectual property protections. A significant contribution by an
individual or group, if it is the basis for compensation, should be appropriately documented.
Companies should base the calculation of royalties payable to a health care professional in exchange for intellectual property
on factors that preserve the objectivity and autonomy of medical decision-making and avoid the potential for improper
influence. For example, a company should not condition royalties paid in exchange for intellectual property on: (1) a
requirement that the health care professional purchases, orders or recommends any use of medical technology of the
company or technology produced as a result of the development project; or (2) a requirement to market the technology upon
commercialization.
Companies are strongly encouraged to consider whether it is appropriate and practicable to exclude from the calculation of
royalties the number of units purchased, used, or ordered by the individual health care professional and/or members of the
health care professional’s practice.
C. Clinical Studies & Research Arrangements
Arrangements that involve clinical research services by a health care professional in return for compensation are also a type of
consulting arrangement, subject to the principles in this section of the Code. The clinical program for which the services are
being provided should fulfill a legitimate research purpose, should have due permissions from the competent authorities, and
should be transparent. A written services agreement should govern these arrangements, and companies should base
compensation on the fair market value of the services provided.
A clinical study agreement typically is entered between a company and a health care professional that is a facility, institution,
or practice group, and compensation for the clinical research services is paid to that entity. An individual health care
professional may act as a study investigator but also provide related services in his or her individual capacity that is outside the
scope of the services covered in the clinical study agreement (e.g., protocol development, delivering education and
presentations on the company’s behalf, etc.). In that case, companies are strongly encouraged to enter a separate consulting
arrangement with that individual health care professional.

9
SECTION III – COMPANY-CONDUCTED PROGRAMS & MEETINGS WITH HEALTH CARE
PROFESSIONALS
Companies have a legitimate need to conduct training and education for health care professionals and to hold other important
business meetings with health care professionals.
A. Company-Conducted Training & Education
Companies have a responsibility to train and educate health care professionals on their medical technologies, the procedures
in which these medical technologies are used, and other related information:
✓ Medical technology may involve complex equipment, devices, and/or sophisticated software platforms that require
technical instruction.
✓ Medical technology may have a new procedure of insertion and deployment technique and may require sufficient
hands-on experience before application on patient.
✓ Medical technology may require analysis of new monitoring parameters.
✓ Medical technology may include breakthrough innovations.
✓ Procedures in which medical technologies are used may be complex and require skilled clinical instruction.
✓ Health care professionals need training and education on disease states and treatment options, patient selection
criteria, clinical treatment standards and outcomes, care pathways, and how medical technologies benefit certain
patient populations, among other important topics.
All of this information contributes to the safe and effective use of medical technology.
Companies should apply the following principles when conducting training and education programs concerning medical
technologies for health care professionals:
• Setting. Companies should conduct live or virtual training and education programs in modest settings that are
conducive to the effective transmission of information. These may include clinical, educational, conference, or other
center of excellence developed by the company for such purposes, either in country, offshore, or other settings such
as hotels or other commercially available meeting facilities. It could also include the health care professional’s
location. Programs providing hands-on technical training and instruction on medical technologies (for example, a
cadaver lab) should be held at training facilities, medical institutions, laboratories, or other appropriate facilities.
• Faculty. Companies should only engage faculty that have the proper qualifications and expertise to conduct the training
or education. This may include health care professionals or qualified company employees (including field sales staff)
who have the technical expertise and experience necessary to perform the training.
• Attendees. Health care professionals must have a legitimate need to attend a company-conducted training or
education program (for example, the need to obtain technical instruction on how to use a new medical technology

10
• Travel & Lodging. See Section VI of the Code for more information on providing travel and lodging to health care
professionals to attend a company-conducted training or education program.
• Meals & Refreshments. See Section VII of the Code for information on providing meals and refreshments to health
care professionals attending a company-conducted training or education program.
• Documentation. Companies are required to maintain documentation of the training to demonstrate that the purpose
of the training has been sufficiently met. Documentation may include copies of the agenda, training content,
photographs, attendees and any other such record.
B. Company Business Meetings
Companies may identify a legitimate need to conduct other types of business meetings with health care professionals to
discuss, for example, medical technology features, sales terms, company service offerings and their impact on health care
delivery, product line offerings, health economics information, feedback and advice on medical technologies or purchase
contract arrangements. Other examples could include plant or facility tours, meetings to demonstrate equipment, or meetings
to explore product development or clinical testing needs.
Companies should apply the following principles when organizing and conducting business meetings:
• Legitimate Need. Companies must have a legitimate need to conduct the meeting. For example, a company may
identify a need to show health care professionals how they make medical technologies, their quality control systems,
or other aspects of their manufacturing processes through a plant tour.
• Setting. Companies may hold meetings at or close to a health care professional’s place of business or facility; another
centralized location; or at a company’s own facility or business centers that may be a more appropriate setting for the
meeting, depending upon the topics discussed. In all instances, the setting for a company-conducted program or
meeting must be conducive to the discussion of relevant information.
• Attendees. Each health care professional in attendance must have an objective, legitimate need to attend a company’s
business meeting.
• Travel & Lodging. See Section VI of the Code for information on providing travel and lodging to health care
professionals attending a company’s business meeting.
• Meals & Refreshments. See Section VII of the Code for information on providing modest meals and refreshments to
health care professionals attending a company’s business meeting.
• Documentation. Companies are required to maintain documentation of the meeting to demonstrate that the purpose
of the meeting has been sufficiently met. Documentation may include copies of the agenda, meeting content,
photographs, attendees and any such record.
11
SECTION IV – EDUCATIONAL & RESEARCH GRANTS, CHARITABLE DONATIONS, COMMERCIAL
SPONSORSHIPS
Companies provide monetary, in-kind, and other contributions to third parties in support of their educational, scientific,
charitable, and research programs.
Companies can support these programs for many valid reasons, such as advancing medical education and training for health
care professionals, raising patient and public awareness on important health care topics, helping underserved or indigent
populations through bona fide charitable programs, or funding independent scientific or clinical research.
A. Supporting Third-Party Programs Through Educational Grants and Commercial Sponsorship
Third-party programs allow companies to support health care professional- and patient-related training and education to
participate in clinical, research and scientific exchanges related to their medical technologies and to demonstrate the safe and
effective use of their products, services and technologies.
Companies should apply the following principles when supporting third-party programs through educational grants and/or
commercial sponsorship:
SUPPORTING
THIRD-PARTY
PROGRAMS
THROUGH
EDUCATIONAL
GRANTS
Companies may provide an educational grant in support of a third-party program directly to the third-
party program organizer or, in some instances, to a training institution, health care professional
association or other entity designated by the third-party program organizer.
A third-party program organizer (or training institution or designee) may use an educational grant in a
transparent manner:
✓ To defray or reduce the costs of conducting the educational components of a third-party
program
✓ To allow health care professionals-in-training (for example, medical and nursing students,
residents, and fellows) to attend the third-party program, provided that the company does not
select or control the selection of the specific health care professionals-in-training who will
benefit
✓ To cover the reasonable compensation, travel, lodging, and modest meals of health care
professionals who serve as bona fide faculty at the third-party program.
✓ To provide health care professionals attending the third-party program with items of value
permissible under the Code, such as modest meals, refreshments, and educational items.
Sales personnel should not control or unduly influence the decision of whether a particular entity will
receive an educational grant or the amount of the grant.
SUPPORTING
THIRD-PARTY
PROGRAMS
THROUGH
When companies provide commercial sponsorship in support of a third-party program, the level of
commercial sponsorship should reflect the fair market value of the benefits received by the company,
such as signage, display/ exhibit space, electricity/ audio-visual equipment/ furniture to be used in
display/ exhibit space or other opportunities to communicate/ display company specific products/

12
• No Support to Individuals. Companies may not provide any contribution (whether monetary or in-kind) directly to an
individual health care professional or pay directly for an individual health care professional’s registration, fees, or
travel or lodging expenses to attend a third-party program.
• Adherence to Program Standards. Companies should adhere to all standards established by the third-party program
organizer or the body accrediting the third-party program, as applicable.
• No Direct Sponsorship. Direct sponsorship of a health care professional to a third-party program is not permitted.
Companies are not permitted to pay, offer to pay or offer to reimburse any expense (e.g., travel, stay, local travel,
honorarium etc.) in support of attendance of health care professionals to third-party programs as faculty. Companies
must also preserve the independence of third-party programs and must not select or influence selection of health care
professional attendance to third-party program. Companies may engage health care professionals under a
professional services agreement for a company-conducted satellite symposium held alongside third-party programs
and may compensate in accordance with fair market value. Travel and lodging should be considered only if the health
care professional is travelling solely for the satellite symposia.
B. Supporting Other Third-Party Programs through Educational Grants
Companies may provide educational grants to training institutions (such as medical schools and teaching hospitals) and to
other third-party entities in support of their legitimate scientific, educational and training programs and other activities. This
includes, but is not limited to, educational grants to support the education and training of health care and medical personnel
(for example, physicians, medical students, residents, fellows, or other health care professionals-in-training or in practice),
patients, government officials and regulators (per approval from respective institutions and as per applicable laws and
applicable service rules), and the selected patient group/public about important health care topics.
Companies may not make an educational grant to individual health care professionals or individual health care professionals-
in-training, and companies may not select or influence the selection of the individual health care professionals who might
benefit from the company’s support.
Sales personnel should not control or unduly influence the decision of whether a particular institution will receive support or
the amount of the support.
C. Supporting Independent Third-Party Research
Supporting third-party research programs and partnering with health care professionals to advance independent research can
provide valuable scientific and clinical information, improve clinical care, lead to promising new medical technologies,
promote improved delivery of health care, and otherwise benefit patients. To help meet these objectives, companies may
provide in-kind or monetary research grants in support of independent research with scientific merit.
• Objectives & Milestones. Companies may provide support for research that has defined goals, objectives, and
milestones. Requests for research grants should be accompanied by clinical protocols that outline these objectives and
milestones. Requests for research grants should also transparently document the nature and scope of the research
COMMERCIAL
SPONSORSHIPS
technologies/ services. Companies may provide commercial sponsorship even if the company determines
not to provide the third-party program organizer with an educational grant.

13
activity, the budget, the approximate duration of the research, and where applicable, the requirements for
independent authorizations or approvals.
• Limitations. Research grants may include in-kind or monetary support for legitimate, study-related, documented
expenses or services and/or reasonable quantities of no-charge product for the limited duration of the research.
• Company Involvement. The recipient of a company’s monetary or in-kind research support should retain independent
control over the research.
• Company Review Processes. Companies should establish controls for reviewing requests for research grants and
ensuring that there is compliance with applicable laws, guidelines and codes and applicable service rules, if any.
• Sales Involvement. Sales personnel should not control or unduly influence the decision of who will receive support or
the amount of the support.
Company-initiated or directed research involving a company’s medical technology (such as clinical study agreements) is
addressed separately in Section II of the Code.
D. Supporting Charitable Programs through Charitable Donations
A company may make monetary or in-kind charitable donations of product or equipment for charitable purposes, such as
compassionate usage, patient or public education.
• Charitable or Philanthropic Mission. Donations should be made for bona fide charitable purposes and should be made
only to charitable organizations or other non-profit entities with bona fide charitable and/or philanthropic purposes.
Companies should exercise diligence to ensure the charitable organization or charitable purpose is bona fide. Relevant
factors to consider may include (1) the entity’s tax status, (2) the entity’s corporate status under local law, (3) whether
the organization has a charitable mission or purpose, and (4) local laws and regulations [for example the Foreign
Contributions (Regulation) Act, 2010 and the Rules made thereunder], among other factors.
• Use of Funds. Companies must require that any donation is used only towards charitable or philanthropic purposes.
• Compassionate Usage. Companies may make charitable donations of product for compassionate usage, provided that
these donations serve exclusively to benefit patients and are permitted under applicable laws [for example, Drugs and
Cosmetics Act, 1940 and the Rules (Medical Devices Rules, 2017, New Drugs and Clinical Trials Rules 2019 & Drugs &
Cosmetics Rules, 1945) made thereunder]. Companies should consider making product donations for compassionate
usage cases contingent upon a hospital’s agreement that no third parties will be billed for the donated product.
• Charitable Events. Companies may not pay for or provide tickets to health care professionals or their spouses or
guests to attend charitable events, such as galas and golf outings.
• Sales Involvement. Sales personnel should not control or unduly influence the decision of whether a particular entity
will receive support or the amount of the support.

14
SECTION V – JOINTLY CONDUCTED EDUCATION & MARKETING PROGRAMS
Companies may partner with health care professionals to jointly conduct education and marketing programs. These programs
serve an important purpose by allowing companies and health care professionals to educate fellow health care professionals
on medical conditions and the range of testing or treatment options available, including the availability of medical technology
and the health care professional’s ability to diagnose or treat related medical conditions.
Companies should apply the following principles:
• There must be a bona fide, legitimate need for the company to engage in the activity.
• Companies should establish controls to help ensure that decisions to engage in these arrangements are not made as
an unlawful inducement. Companies should also require health care professionals participating in these arrangements
to comply with company guidelines and local regulatory guidelines on providing all relevant regulatory and clinical
information related to the product’s labeling, safety, quality, instructions for use, performance, safety, side-effects
and potential adverse-effects among other controls. Such programs shall be subject to the local law including but not
limited to registration of the product by the relevant regulatory authority.
• Jointly conducted programs should be balanced to disseminate and exchange information to the health care
professional and the range of services offered by the industry, the treatment of related medical conditions in that
disease area as well as the range of technologies and services which are available in the competitive market.
• The company and the health care professional should serve as bona fide partners in the program. The arrangement
should be documented in a written agreement that describes the purpose of the arrangement and the roles,
responsibilities, and mutually agreed contributions of each party, including payment of costs.

15
SECTION VI – TRAVEL, LODGING & VENUE
There may be educational/training programs or other scientific meetings for which a company determines it is appropriate to
pay for health care professionals’ travel and lodging costs. This section of the Code provides companies with guidance on
paying for a health care professional’s travel and lodging costs. Companies should apply the following principles:
• Legitimate Need. There must be objective, legitimate reasons that support the need for out-of-town travel, such as
the need to deliver training and education concerning medical technologies (for example, the non-availability of health
care professionals who may not require travel, established expertise in a particular field/therapy/practice, the inability
to effectively deliver the content of the program through means other than an in-person meeting, or the need to
demonstrate equipment). Companies are encouraged to document the legitimate need for travel.
• Modest & Reasonable. Travel and lodging accommodations and costs must be modest and reasonable under the
circumstances. Companies are encouraged to establish controls on the appropriate class of travel service and the
appropriate level of lodging accommodations.
• Travel Time & Destination. Companies are also encouraged to ensure criteria’s are established and taken into
consideration while making such arrangements for healthcare professionals (for example, travel dates to be -/+ 24
hours from the start and end of the event date(s), respectively, or travel to and from the place of the healthcare
professionals’ official residence/practice unless there is a justified need to make arrangements from some other
place).
• Guests. Companies may not pay for or otherwise subsidize the travel or lodging of spouses or guests of health care
professionals or for any other person who does not have a bona fide professional interest in the information being
shared at the company’s meeting.
• Personal Travel & Lodging. Companies may not pay for a health care professional’s personal travel or lodging costs
that are not connected to such company-conducted program.
• Setting. The setting for a company-conducted program or meeting of health care professionals should always be
conducive to the exchange of information, suit the particular purpose of the program/ training/meeting, and should
not be the main attraction of the event. Companies should consider the following principles when choosing a setting:
✓ The setting should be in a business/ clinical environment as deemed suitable for the purpose, centrally located
and easily accessible (for example, considering proximity to airports and highways) in relation to the place of
origin of the invited participants.
✓ Companies should not select a setting because of its entertainment or recreational facilities (considering, for
example, the season or time of year of the event).
✓ Companies should avoid top category or luxury hotels or resort facilities without an appropriate justification.
• Other Laws. Companies should be aware that other laws or regulations may apply to paying for health care
professionals’ travel and lodging, including potentially more restrictive local laws, institutional rules and service rules
applicable to the health care professionals.

16
SECTION VII – PROVIDING MODEST MEALS & REFRESHMENTS TO HEALTH CARE PROFESSIONALS
Companies may occasionally provide health care professionals with modest meals and refreshments subject to the following
principles:
• Purpose. The meal or refreshments should be subordinate in time and in focus to the bona fide discussion and
presentation of scientific, educational, or business information. Companies should only provide meals and refreshments
in a manner conducive to the presentation or discussion of such information. The meal or refreshments should not be
part of an entertainment or recreational event.
• Setting & Location. Meals and refreshments should be provided in a setting that is conducive to bona fide scientific,
educational, or business discussions. This may include, for example, the health care professional’s place of business, the
company’s office or an off-site space that is conducive to the discussion, such as a restaurant or business center.
• Participants. Companies may provide a meal or refreshments only to health care professionals who actually attend and
have a bona fide purpose for attending the meeting.
Companies may not provide a meal or refreshments:
✓ For an entire office staff where everyone does not attend the meeting;
✓ If a company representative is not present; or
✓ For guests of health care professionals or for any other person who does not have a bona fide professional interest in
the information being discussed at the meeting.
• Documentation. Companies should establish controls to ensure the purpose and execution of the meeting is reasonably
documented.
• Frequency. Companies should establish controls to reasonably monitor and track the frequency of meetings with health
care professionals involving meals and refreshments.

17
SECTION VIII – EDUCATIONAL & PATIENT BENEFIT ITEMS; PROHIBITION ON GIFTS
Companies may occasionally provide modest, appropriate educational items to health care professionals that benefit patients
or serve a genuine educational function for health care professionals. Educational items can include but are not limited to
product manuals and anatomical models.
Companies may not provide gifts to health care professionals. This means that companies may not provide health care
professionals with the following:
✓ Items that the health care professional (or his or her family members, office staff, or friends) can use for non-
educational or non-patient-related purposes (for example, office supplies, scrubs, a tablet, smart phone, laptop, or
other mobile device capable of personal use)
✓ Non-educational promotional items, even if the item is of minimal value, related to the health care professional’s
work, or for the benefit of patients (for example t-shirts, hats, mugs, and other items with a company or product name
or logo)
✓ Gifts such as wine, gift baskets, holiday gifts or cash or cash equivalents (for example, gift cards)
Other important principles include:
✓ Any item given to a health care professional’s staff should be treated as though it is given to the health care
professional and is subject to all applicable provisions of the Code.
✓ Companies may not raffle or give away an item that it could not otherwise give a health care professional under the
Code.

18
SECTION IX – PROHIBITION ON ENTERTAINMENT & RECREATION
Companies should not provide or pay for any entertainment or recreational event for a health care professional, their staff or
their family.
Some examples of entertainment and recreational activities include, among others, theater, live comedy or musicals, sporting
events, golf, skiing, cruises, spas, or vacation trips.
This prohibition applies regardless of (1) the value of the activity; (2) whether the company engages the health care
professional as a consultant; or (3) whether the entertainment or recreation is secondary to an educational purpose.

19
SECTION X – PROVISION OF HEALTH ECONOMICS & REIMBURSEMENT INFORMATION
As medical technologies become increasingly complex, so do payor coverage and reimbursement policies. Patient access to
necessary medical technology depends on health care professionals and/or patients having timely and complete coverage,
reimbursement, and health economic information. To promote patient access to medical technologies:
• Companies may provide this information to health care professionals regarding its medical technologies if it is
accurate and objective.
• Companies may also collaborate with health care professionals, health economists, reimbursement experts and
organizations representing their interests to achieve government and commercial payor coverage decisions,
guidelines, policies, and adequate reimbursement levels that allow patients to access its medical technologies.
Permissible activities involving the provision of coverage, reimbursement, and health economic information may include, but
are not limited to:
• Identifying the clinical value of the company’s medical technologies and the services and procedures in which they are
used.
• Collaborating with health care professionals, their professional organizations, health economists, reimbursement
experts and patient groups to conduct joint advocacy on coverage, reimbursement, and health economics issues.
• Supporting health care professionals and their professional organizations in developing materials and otherwise
providing direct or indirect input into payor coverage and reimbursement policies.
• Promoting accurate payor claims by providing accurate and objective information and materials to health care
professionals regarding the company’s medical technologies, including identifying coverage, codes, and billing options
that may apply to those medical technologies or the services and procedures in which they are used.
• Providing accurate and objective information about the economically efficient use of the company’s medical
technologies, including where and how they can be used within the continuum of care.
• Providing information related to the company’s medical technologies and available reimbursement and associated
costs.
• Providing information relating to changes in coverage or reimbursement amounts, methodologies and policies and the
effects of such changes to help a health care professional in the decision to use or recommend use of the company’s
medical technologies.
• Providing accurate and objective information designed to offer technical or other support intended to aid in the
appropriate and efficient use or installation of the company’s medical technologies.
• Providing accurate pricing and payment documentation on the company’s medical technologies to the hospital and
maximum retail prices to the relevant authorities and health care professionals.

20
• Facilitating patient access to a company’s medical technologies by providing health care professionals with assistance
in obtaining patient coverage decisions from payors, including providing information on payor policies and training on
procedures for obtaining prior authorization, providing sample letters and information on medical necessity and
appeals of denied claims.
In addition, at the request and recommendation of a health care professional to facilitate patient access to the
company’s medical technology and subject to appropriate privacy safeguards, the company may assist the patient by
facilitating the preparation and submission of requests for coverage determinations, prior authorizations, pre-
certifications and appeals of denied claims relating to a company’s own medical technology, however, such assistance
should not be provided as an unlawful inducement.
Companies may not interfere with a health care professional’s independent clinical decision making or provide coverage,
reimbursement, and health economics support as an unlawful inducement. For example, companies should not provide free
services that eliminate an overhead or other expense that a health care professional would otherwise have incurred as part of
its business operations. Further, companies should not suggest mechanisms for billing for services that are not medically
necessary, or for engaging in fraudulent practices to achieve inappropriate payment.
If a company comes across information on any patients’ medical records while collaborating with a health care professional or
patient in obtaining reimbursement, the company should maintain the privacy of the data as per applicable laws, regulations,
guidelines, codes and applicable global standards. Similarly, the company should fulfil its other regulatory obligations such as
initiating field corrective measures and/or informing the relevant regulatory authority.

21
SECTION XI – DEMONSTRATION, EVALUATION & CONSIGNED PRODUCTS
A. Demonstration & Evaluation Products
Providing products (approved medical technologies) to health care professionals at no charge for evaluation or demonstration
purposes can improve patient care, facilitate the safe and effective use of products, enhance patient awareness, and educate
health care professionals regarding the use of products. Under certain circumstances including tendering processes, a
company may provide reasonable quantities of products to health care professionals at no charge to allow health care
professionals to assess the appropriate use, safety and performance of the product.
Company products that may be provided to health care professionals for evaluation include single use (for example, samples
of consumable or disposable products) and multiple use products (sometimes referred to as capital equipment).
Company products provided for evaluation are typically expected to be used in patient care. Companies should provide health
care professionals with appropriate documentation to allow the health care professional to address any reimbursement
reporting obligations, including providing information on the no-charge status of these products.
• Single Use/Consumables/Disposables. The number of single use products provided at no charge should not exceed
the quantity reasonably necessary for the adequate evaluation of the products.
• Multiple Use/Capital. Multiple use products provided without transfer of title for evaluation purposes should be
furnished only for a period of time that is reasonable under the circumstances to allow an adequate evaluation.
o The length of time necessary for a health care professional to evaluate a multiple use product can vary among
products and may depend on the frequency of anticipated use, the duration of required training, the number
of health care professionals who need to evaluate the product, the length of time needed to evaluate
different product features, and other considerations such as local laws, standards and regulations.
o The terms of evaluation of such multiple use products should be set in advance in writing, specifying the
length of the evaluation period and documenting legitimate reasons for products that would not be returned
within the evaluation period.
o Companies should retain title to multiple use products during the evaluation period and should have a process
in place for promptly removing multiple use products from the health care professional’s location at the
conclusion of the evaluation period unless the health care professional purchases or leases the products.
• Demonstration. Company demonstration products are sometimes unsterilized single use products or mock-ups that
are used for health care professional and patient awareness and education. For example, a health care professional
may use a demonstration product to show a patient the type of device that will be implanted in the patient.
o Company demonstration products are typically identified as not intended for patient use through designations
like “Free Sample - NOT FOR SALE” or “Not for Human Use” on the product, the packaging, or documentation
that accompanies the product. Sterilized products may be used in live demonstration only if done in an
educational setting with company controls in place.

22
• Documentation. Company should maintain a system of control in respect of such demonstration products including
traceability (such as product name, product code, quantity, batch number, lot number and, name of the healthcare
professional to whom such demonstration products are given etc.).
B. Consigned Products
Consigned products are medical technologies (a) that a company provides to a health care professional for use in and storage
at the health care professional’s patient care setting and (b) to which the company retains title until the product is used.
• Consignment arrangements should generally be subject to an agreement that addresses the terms of consignment, for
example, the number of products, any requirements to segregate consigned products from other products, and
storage conditions and responsibility of ensuring the storage conditions of the products.
• Companies are encouraged to consider implementing appropriate controls. This could include (among other
measures) taking periodic inventory of consigned devices for purposes such as billing and restocking; reconciling
discrepancies between the company’s records and the number of products used or verified during inventory; and
return or removal of expired product.

23
SECTION XII – COMPANY REPRESENTATIVES PROVIDING TECHNICAL SUPPORT IN THE CLINICAL
SETTING
Company representatives may play an important role in the clinical setting by providing technical support on the safe and
effective use of medical technology. Some examples include:
• Company representatives may need to explain how a medical technology’s unique settings and technical controls
function and may make recommendations.
• Company representatives may assist the clinical/operating room team to ensure that the appropriate range of
necessary devices and accessories are available during a procedure, especially when dealing with medical technology
that involves multiple devices and/or accessories.
Companies should apply the following principles:
1. Company representatives should enter and be present in the clinical setting only at the request of and under the
supervision of a health care professional.
2. Company representatives should be transparent that they are acting on behalf of the company in a technical support
capacity only. The primary patient care responsibility lies exclusively with the health care professional.
3. Company representatives should ensure that the professional autonomy of the health care professional and/or
autonomy of the medical institution is maintained by not interfering with a health care professional’s independent
clinical decision-making.
4. Company representatives should comply with applicable hospital or facility policies and requirements, including
patient privacy and credentialing requirements as per the applicable laws, guidelines and codes.
5. A company’s technical support should not cover an overhead or other expense that the health care professional would
otherwise incur while providing patient care. For example, a company representative providing technical support for a
procedure with regard to the company’s medical technology may not function as a surgical technician for a hospital.
6. Company representatives may provide their personal contact details where required if the product requires consistent
monitoring, programming or adjustment, but such support can only be provided per the health care professional’s
direction.

24
SECTION XIII – RELATIONSHIPS WITH THIRD-PARTY INTERMEDIARIES
Companies are encouraged to adopt a third-party intermediary management compliance program in addition to an overall
compliance program. Taking into account a variety of risk-based factors, as well as local applicable laws, such programs should
include the following elements:
1. Written Anti-Bribery Policy/Procedure: Companies should adopt and implement internal policies prohibiting all forms
of bribery by any person or entity acting on a company’s behalf, including company personnel, third-party
intermediary representatives, health care professionals and other agents. Such policies should include more detailed
measures for common risk areas such as travel, gifts, hospitality, entertainment, grants or donations, research, and
capital equipment. Companies should consider communicating to health care professionals and other stakeholders
their ethical business practices concerning third-party intermediaries.
2. Risk Assessment: Companies should evaluate the risk profile for proposed and utilized third-party intermediary
arrangements including, for example:
• Companies should assess (1) the local risk through published corruption indices as well as specific risk profiles
of planned or utilized Third-party intermediaries; (2) international, national and local legal requirements, (3)
information from third-party intermediaries for potentially unusual arrangements, such as unusually high
commissions paid to sub-intermediaries, high degree of interaction with government officials or health care
professionals associated with Government hospitals, marketing budgets, health care provider corporate
affiliation or ownership, and/or off-shore payment accounts, and (4) information available from public sources
or employees for potential issues associated with a third-party intermediary.
• Third-party intermediaries should (1) support companies’ risk assessments prior to and throughout
engagement in activities conducted on the company’s behalf, (2) assess and comply with international,
national and local legal requirements, (3) disclose potentially unusual arrangements, and (5) maintain accurate
records for review.
3. Diligence Program: Companies should establish a risk-based, pre-engagement and renewal due diligence program to
identify, prevent, and mitigate risks relating to the market in which the third-party intermediaries is engaged to
operate, as well as any specific activities the third-party intermediary deploys on behalf of the company. Companies
are encouraged to engage with local industry associations to advance compliance training and best practices sharing.
4. Written Contract: Companies should reach contract terms with third-party intermediaries that implement anti-
corruption policies including but not limited to:
a. Compliance with international, national and local laws, ethical principles, and company policies;
b. The ability to conduct independent audits and monitoring, including access to relevant books and records;
c. The ability to terminate an engagement for failure to comply with international and local laws, ethical
principles, and company policies; and
d. Diligence rights upon renewal.
5. Training and Education: Companies should undertake initial and provide regular training and education for relevant
third-party intermediary personnel on international and applicable national anti-corruption and anti-bribery laws, data
privacy principles and other laws and regulations that may be relevant for third-party intermediaries to conduct their

25
business per ethical principles and company policies. Training should be conducted in the language most appropriate
to the audience.
6. Monitor/Audit: Companies are encouraged to undertake risk-based, routine monitoring, auditing, and other
assessments of their relationship for compliance with international and applicable national and local laws, ethical
principles, and company policies as well as relevant contract terms.
7. Appropriate Corrective Action: Companies should take appropriate corrective action, consistent with applicable
international and applicable national and local laws, if a third-party intermediary representative fails to comply with
such international and applicable national and local laws, ethical principles, company policies, relevant contract terms,
or engages in other impermissible or unethical conduct.
