
1
Interpretation Guide

2
AVISE Interpretation Guide
SLE Associated Markers
Marker (method) Associated Disease Sensitivity Interpretation
EC4d (FACS) Systemic Lupus
Erythematosus (SLE)
46%
1
Cell-Bound Complement Activation Products (CB-CAPs) EC4d & BC4d are measures of
classical complement activation. CB-CAPs are primarily associated with SLE with 66%
of patients having one or both CB-CAPs elevated
1
. EC4d signicantly correlates with
uctuations in SLE disease activity
2
.
BC4d (FACS) SLE prognostic 53%
1
C3 (IT) SLE 33%
1
C3/C4 proteins are integral components of the complement system. Abnormally low
concentrations of C3/C4 associate with SLE primarily with 44% of SLE patients having one
or both proteins abnormally low.
C4 (IT) SLE prognostic 32%
1
Anti-C1q IgG
(ELISA)
Lupus Nephritis/
disease activity
58%
4
Antibodies against the complement protein C1q are found in 58% of SLE patients with
active lupus nephritis. Anti-C1q levels associate with renal activity
2
. Low levels of anti-
C1q antibodies have been found in up to 8% of patients with other diseases, such as
infection and normal healthy individuals.
Anti-dsDNA IgG
(ELISA and IFA)
SLE 33%
1
Anti-dsDNA antibodies provide high positive predictive value due to high specicity for
SLE. In the AVISE Lupus algorithm, patients are initially screened with an ELISA assay, and
all patients testing positive are reexed to Crithidia luciliae IFA testing for conrmation.
Anti-dsDNA
(CIA)
SLE/disease activity 46%
24,25
The anti-dsDNA by CIA method measures quantitative levels of anti-double stranded
deoxyribonucleic acid with a superior correlation to disease activity compared to other
methods.
Anti-Nuclear
Antibodies IgG
(ANA)
(ELISA and IFA)
Autoimmune Diseases 89% in SLE
1
ANA has high negative predictive value for ruling out SLE due to its high sensitivity for
SLE. However, ANA is found in many autoimmune disorders and a signicant proportion
of apparently healthy individuals.
Anti-Ribosomal
P IgG
(ELISA)
Neuropsychiatric Lupus 9%
5
Antibodies against ribosomal-P are highly specic for SLE and can be present in anti-
dsDNA or anti-Sm negative patients. Anti-Ribosomal-P antibodies have been shown to
associate with neuropsychiatric SLE manifestations.
Anti-Smith IgG
(ELISA)
SLE 14%
1
Anti-Smith antibodies are highly specic, but comparatively low sensitivity marker for
SLE. One of the ACR criteria for SLE.
ENA Markers
Marker (method) Associated Disease Sensitivity Interpretation
Anti-CENP IgG
(ELISA)
CREST Syndrome 20-60%
6
Antibodies to (CENP) protein-B are found in 20-60% of patients with CREST syndrome, a
limited form of scleroderma.
Anti-Jo-1 IgG
(ELISA)
Polymyositis/
Dermatomyositis (PM/
DM)
20-30%
7
Marker to aid diagnosis of PM/DM. Found in about 25% of patients with PM/DM.
Anti-RNP70 IgG
(ELISA)
Mixed Connective
Tissue Disease (MCTD)
90%
8
RNP70 is a 70 kDa protein of the U1-snRNP complex. RNP70 antibodies are highly
sensitive for MCTD.
Anti-Scl-70 IgG
(ELISA)
Scleroderma 28-70%
9
Up to 70% of scleroderma patients are positive. Anti-Scl70 is highly specic for diffuse
cutaneous scleroderma and associates with interstitial lung disease (ILD).
Anti-Ro52 IgG
(CIA)
Myositis, SLE,
Sjögren’s Syndrome &
Scleroderma
20-70%
10
Ro52 antibodies are found in multiple autoimmune conditions. Anti-Ro52 has been
shown to associate with interstitial lung disease (ILD) in patients with Sjögren’s syndrome
or scleroderma.
Anti-Ro60 IgG
(CIA)
SLE, Sjögren’s
Syndrome, Myositis &
Scleroderma
20-65%
10
Ro60 antibodies are found in multiple autoimmune conditions. Anti-Ro60 is commonly
found in both SLE and Sjögren’s syndrome.
Anti-RNA PoI
III IgG
(ELISA)
Systemic Sclerosis 5-22%
11
Antibodies against RNA polymerase III are specic for systemic sclerosis often found in
the absence of anti-Scl70 and anti-CENP antibodies. RNA Pol III antibodies associate with
diffuse cutaneous scleroderma.
Anti-SS-B/La IgG
(ELISA)
Sjögren’s Syndrome 39%
1
Anti-La antibodies are a low sensitivity but highly specic marker for Sjögren’s Syndrome.
Anti-U1-RNP IgG
(ELISA)
MCTD 95-99%
8
Anti-U1-RNP antibodies are highly sensitive for MCTD. Absence of anti-U1-RNP antibodies
is used to rule out MCTD.
Anti-Histone IgG
(ELISA)
Drug-Induced Lupus
(DIL)
95%
12
Up to 95% of drug-induced lupus and 50% of SLE patients exhibit elevated levels of
histone antibodies. Histone antibodies have also been found in RA, DM, and SS placing
added importance on clinical presentation.
AVISE CTD
AVISE APS
AVISE Lupus
SLE Monitor
SLE Prognostic
AVISE Vasculitis
3
Marker (method) Associated Disease Sensitivity Interpretation
RA Markers
Anti-Cyclic
Citrullinated
Peptide IgG (ELISA)
Rheumatoid
Arthritis (RA)
70-90%
13
Antibodies to Cyclic Citrullinated Peptides (CCP) aid in the diagnosis of Rheumatoid
Arthritis (RA). Anti-CCP antibodies are highly specic for RA. Anti-CCP is included in the
AVISE Lupus algorithm to help with the differential diagnosis of RA vs. SLE.
Anti-Carbamylated
Protein IgG
(ELISA)
RA 33%
14
Anti-CarP antibodies serve as a marker of more severe prognosis in RA, independent of
anti-CCP or RF status. Studies have shown anti-CarP found in early RA associates with
future erosive damage. The clinical signicance of positive anti-CarP in the absence of
RA has not been established.
Rheumatoid Factor
IgM & IgA (ELISA)
RA 70-90%
13
Quantitative measurement of IgM and IgA rheumatoid factors (RF) to aid in the
diagnosis of rheumatoid arthritis. Presence of IgA RF isotype may be associated with
more severe RA prognosis.
APS Markers
Marker (method) Associated Disease Sensitivity Interpretation
Anti-β2-
Gylcoprotein
I IgG, IgM & IgA
(ELISA)
Antiphospholipid
Syndrome (APS)
45%
15
Antibodies to Beta 2 Glycoprotein 1 (ß 2 GP1) exhibit higher specicity than anti-
cardiolipin assays. In 3-10% of APS patients, ß2 GP1 antibodies may be the only
positive test. Positive results should be conrmed after 12 weeks to ensure persistency
of antibodies. IgA ß2 GP1 antibodies are less common than IgG or IgM and can occur
in isolation.
Anti-Cardiolipin
IgG, IgM & IgA
(ELISA)
APS 97%
16
Antibodies to cardiolipin are present in SLE patients (30-40%) and APS. Prevalence of
cardiolipin in APS is high but specicity for APS is lower than other anti-phospholipid
antibodies. Positive results should be conrmed after 12 weeks.
Anti-
Phosphatidylserine
/Prothrombin
(PS/PT) IgM & IgG
(ELISA)
APS 22-37%
3
Anti-PS/PT antibodies are markers for APS that have been found to signicantly
correlate with lupus anticoagulant (LAC)
16
. Unlike LAC, anti-PS/PT testing is unaffected
by anti-coagulant therapy.
Thyroid Markers
Marker (method) Associated Disease Sensitivity Interpretation
Anti-Thyroglobulin
IgG (ELISA)
Hashimoto’s Thyroiditis
& Graves’ Disease
60-85%
17
Anti-thyroglobulin antibodies are found in 60-85% of patients with Hashimoto’s
thyroiditis and 30-80% of patients with Graves’ disease.
Anti-Thyroid
Peroxidase IgG
(ELISA)
Hashimoto’s Thyroiditis
& Graves’ Disease
71-97%
17
Anti-thyroid peroxidase antibodies are found in > 90% of patients with Hashimoto’s
thyroiditis & 71-97% of patients with Graves’ disease. Over 95% of thyroiditis patients
have Thyroglobulin IgG and/or Thyroid peroxidase antibodies.
Vasculitis Markers
Marker (method) Associated Disease Sensitivity Interpretation
ANCA
(IFA)
ANCA associated
vasculitis
77%
19
The c-ANCA pattern produces a granular cytoplasmic pattern with interlobular
accentuation on ethanol xed neutrophils. c-ANCA patterns are associated with
necrotizing segmental glomerulonephritis and GPA
19, 20
.
85%
19
The p-ANCA pattern produces perinuclear staining with or without nuclear extension.
The p-ANCA pattern is commonly detected in patients with MPA and about 40% of
patients with EGPA
20, 24
.
Anti-PR3 IgG
(CIA)
Granulomatosis with
Polyangiitis (GPA)
81%
19
Anti-PR3 antibodies are primarily associated with GPA and to a lesser extent, found in
MPA (10%) and EGPA
18, 19
. However, anti-PR3 antibodies can also be seen in connective
tissue disease, IBD, some infections, malignancy, and as a reaction to drugs. Therefore,
results should be interpreted with care in light of the clinical ndings and workup
21
.
Anti-MPO IgG
(CIA)
Microscopic
Polyangiitis (MPA)
85%
19
Anti-MPO antibodies are primarily associated with MPA and to a lesser extent, found in
GPA (6%) and EGPA
18,19
. However, anti-MPO antibodies can also be seen in connective
tissue disease, IBD, some infections, malignancy, and as a reaction to drugs. Therefore,
results should be interpreted with care in light of the clinical ndings and workup
21
.
Anti-GBM IgG
(CIA)
Goodpasture’s
Syndrome (GPS)
96%
22
Anti-GBM antibodies are often associated with Goodpasture’s disease and anti-GBM
nephritis
22
. A signicant proportion of patients with anti-GBM disease are also positive
for ANCA.
AVISE Interpretation Guide
AVISE CTD
AVISE APS
AVISE Lupus
SLE Monitor
SLE Prognostic
AVISE Vasculitis
4
AVISE HCQ
A test to aid in assessing adherence to HCQ and individual exposure
to HCQ as measured in whole blood.
HCQ Level Interpretation & Consideration
Therapeutic (>1000 ng/mL) Level associated with clinical efcacy.
HCQ is likely absorbed effectively.
Sub-therapeutic (200-1000 ng/mL) Patient could be partially adherent to therapy.
Patients with HCQ lower than 1000 ng/mL can
be at greater risk for disease are.
Underexposed (<200 ng/mI) Patient is likely non-adherent to HCQ therapy.
AVISE MTX
A test to aid in assessing adherence to MTX and individual exposure to
active MTX metabolites in red blood cells.
MTXPG Level Interpretation & Consideration
Therapeutic (>60 nmol/L) Patient is metabolizing MTX effectively.
Level is consistent with clinical efcacy.
Intermediate (20-60 nmol/L) Patient may need more exposure to MTX.
Sub-therapeutic (<20 nmol/L) Patient may not be metabolizing MTX effectively
or patient may be non-adherent with therapy.
AVISE Interpretation Guide
FACS: Fluorescence-Activated Cell Sorting
IFA: Immunouorescence
Methodology Denitions:
ELISA: Enzyme -Linked Immunosorbent Assay
CIA: Chemiluminescent Immunoassay
IT: Immunoturbidimetry
5
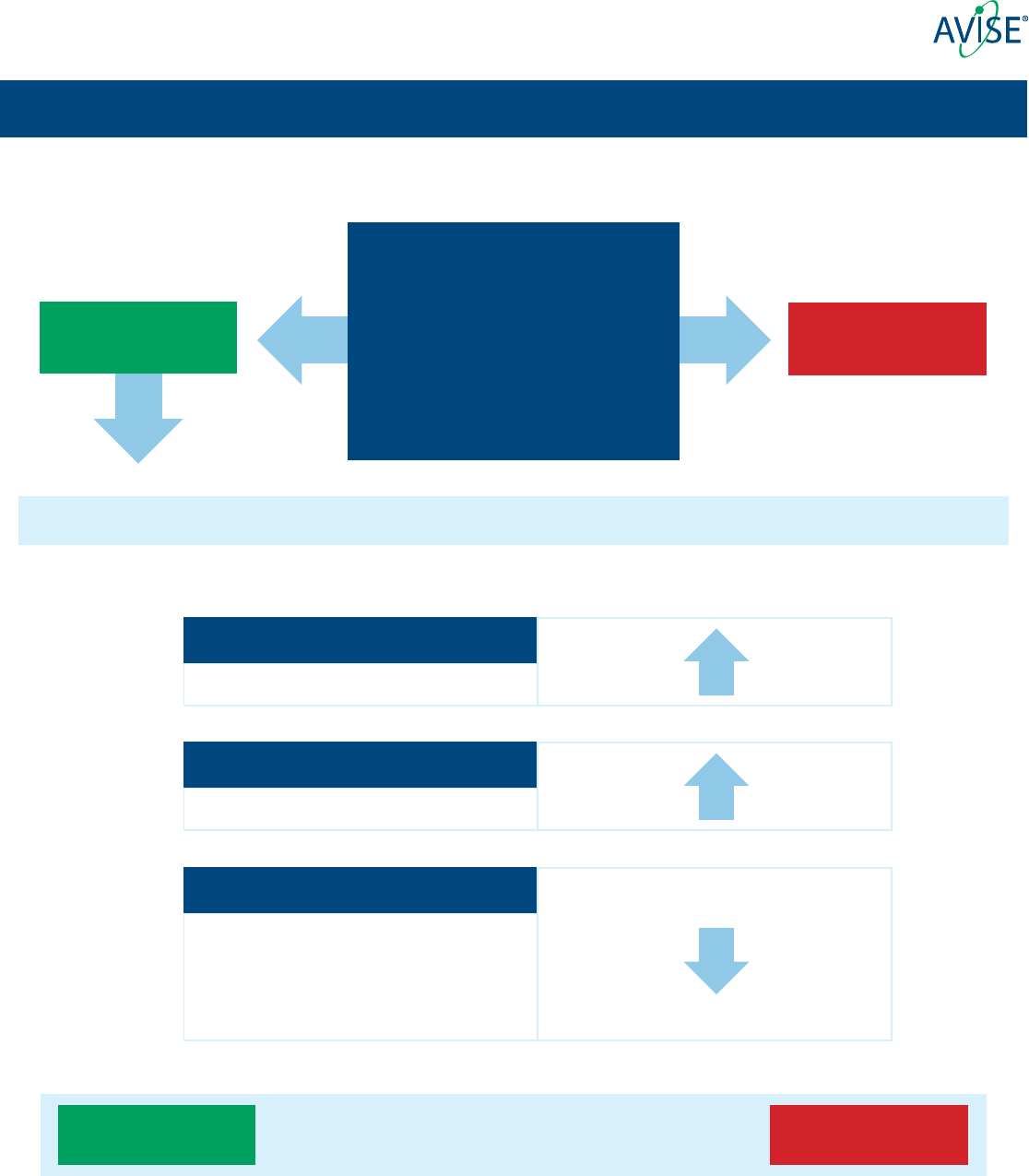
A deeper look at the AVISE® Lupus two tier algorithm
Tier 1 criteria is highly specic for SLE
TIER 1
TIER 2
Any of the Following:
Anti-Sm >10 U/mL
EC4d >75 Net MFI
BC4d >200 Net MFI
Anti-dsDNA ≥ 302 U/ML
If Tier 1 is Negative move to Tier 2
When levels are elevated Impact on Index
(Positive anti-dsDNA ELISA Conrmed
by Crithidia)
Negative
Negative
Positive
Positive
ANA Component
ELISA
CB-CAPs Component
EC4d & BC4d
Specicity Component
Anti-CCP
Anti-SS-B/La
Anti-CENP
Anti-Scl-70
Anti-Jo-1
+
_
+
< -0.1 Index Value >0.1
Tier 2 generates an index value based on the following components
• Level of ANA ELISA result (negative, positive, strong positive)
• Measurement of classical complement activation (CB-CAPs component) measured on a continuous scale
• Presence of auto-antibodies specic to other autoimmune CTDs
AVISE Interpretation Guide
6
Order ID
Identifier Received
Created
Collected
Provider
Susan S.
739814
Exagen Provider MD
12/22/2022
541163
Exagen ID
12/23/2022
PatientSpecimen
Received
Test Order
Reported
Female - 01/01/1996
Gender - DOB
Sample,
12/23/2022
12/28/2022
Approved by:
Results were obtained using flow cytometry for complement C4d fragment bound to erythrocytes (EC4d) and B-lymphocytes (BC4d). Autoantibodies were determined using
solid phase immunoassays. ANA was determined by indirect immunofluorescence and solid phase assays. ANA by solid phase assay was used for the index calculation. In a
study of 794 subjects comprising 304 SLE patients, 285 patients with other rheumatic diseases and 205 normal healthy controls, positivity for Tier 1 markers (anti-dsDNA,
confirmed using Crithidia, anti-Sm or elevated EC4d and BC4d) was associated with a sensitivity of 46% and a specificity of 97%. Among the 440 subjects negative in Tier 1, a
positive index score composite of ANA (by ELISA), EC4d/BC4d and positivity for anti-citrullinated peptide antibodies, SS-B/La, CENP, Jo-1 or Scl-70 resulted in sensitivity of
62% for SLE and specificity of 89%. Two tier combination yielded 80% sensitivity for SLE and 86% specificity for other rheumatic diseases (98% specificity vs. healthy).
Tier 1 Analytes
Tier 2 Analytes
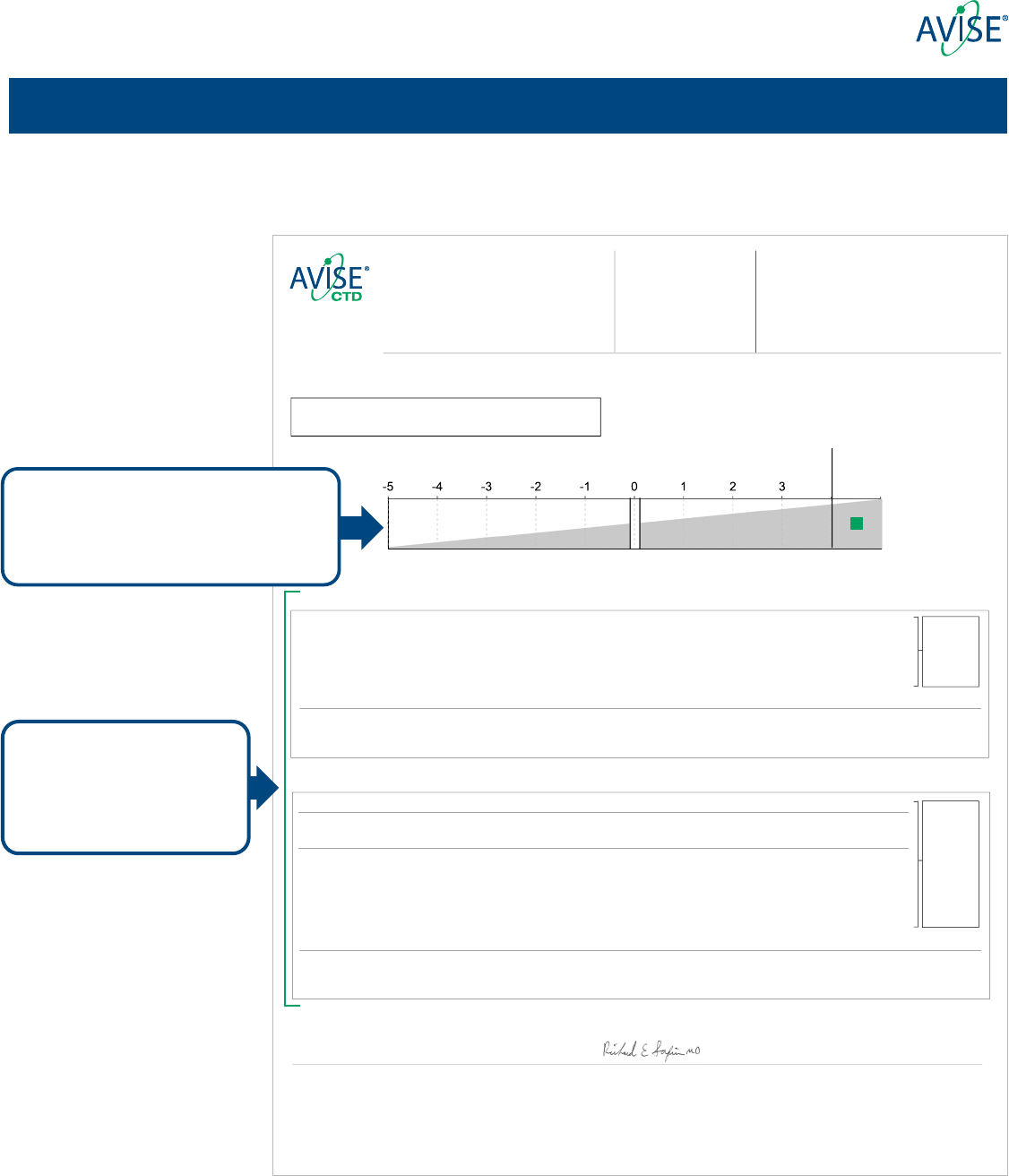
AVISE Lupus Result:
Negative PositiveIndeterminate
12Page of
AVISE CTD Test Report
Tier 1
Positive
CB-CAP: EC4d - Erythrocyte-bound C4d
CB-CAP: BC4d - B-lymphocyte-bound C4d
<61 - Negative | 61-200 - Positive | >200 - Strong Positive
<15 - Negative | 15 -75 - Positive | >75 - Strong Positive
Net MFI
Net MFI
Anti-dsDNA IgG
<201 - Negative | 201-<302 - Equivocal |≥302 - Positive
IU/mL
Anti-Smith IgG
<7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
ANA IgG
<20 - Negative | 20-<60 - Positive | ≥60 - Strong Positive
Units
Anti-SS-B/La IgG
<7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Anti-Scl-70 IgG
<7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Anti-Centromere Protein B (CENP) IgG
<7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Anti-Jo-1 IgG
<7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Anti-CCP IgG U/mL
<7 - Negative | 7-10 - Equivocal | >10 - Positive
Tier 1
Assessment
<61 - Negative | 61-200 - Positive | >200 - Strong Positive
<15 - Negative | 15-75 - Positive | >75 - Strong Positive
Net MFI
Net MFI
Tier 2
Assessment
Value Interpretation Reference Range
Value Interpretation Reference Range
Confirmation by Crithidia luciliae
Note:
Note:
CB-CAP: EC4d - Erythrocyte-bound C4d
CB-CAP: BC4d - B-lymphocyte-bound C4d
The Tier 1 result is associated with an increased likelihood of SLE and is the product of the following analyte values meeting the Tier 1
criteria: Anti-dsDNA, confirmed by Crithidia IFA
Tier 1 Positive
413.00 POSITIVE
Negative
Negative
POSITIVE
1.0
8
125
Positive
Not
assessed
due to
Tier 1
Positive
8
125
Negative
POSITIVE
1.0
1.0
1.0
1.0
1.0
STRONG POSITIVE>150
Negative
Negative
Negative
Negative
Negative
Positive anti-dsDNA confirmed by Crithidia
Date:Richard Safrin, MD 12-28-2022
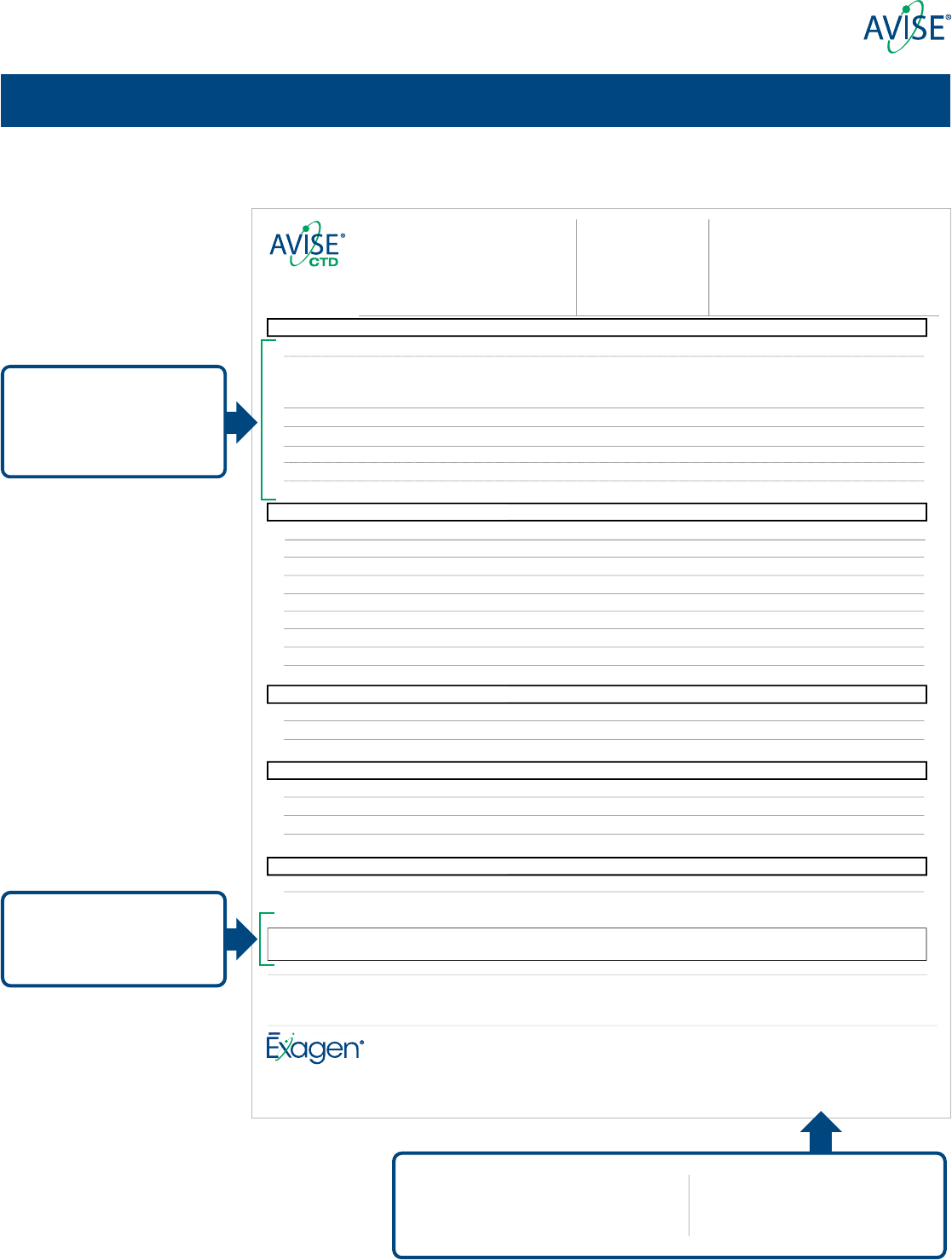
AVISE® CTD and AVISE Lupus result report
Analytes included in Tier 1
and Tier 2 along with respective
assessments are reported in
two distinct sections
The result for the AVISE Lupus algorithm is
featured rst, plotted along a gradient of
increasing likelihood for presence of SLE
AVISE Interpretation Guide
7
Order ID
Identifier Received
Created
Collected
Provider
Susan S.
Exagen Provider MD
739814
12/22/2022
541163
Exagen ID
12/23/2022
PatientSpecimen
Received
Test Order
Reported
Female - 01/01/1996
Gender - DOB
Sample,
12/23/2022
12/28/2022
Value Interpretation
SLE-Associated Analytes
ANA IgG Units
ELISA: <20 - Negative | 20-<60 - Positive | ≥60 - Strong Positive
ANA by HEp-2
IFA: <1:80 - Negative | ≥1:80 - Positive
Titer:
Cytoplasmic Pattern:
CB-CAP: EC4d - Erythrocyte-bound C4d Net MFI
FACS: <15 - Negative | 15-75 - Positive | >75 - Strong Positive
CB-CAP: BC4d - B-lymphocyte-bound C4d Net MFI
FACS: <61 - Negative | 61-200 - Positive | >200 - Strong Positive
Other Autoimmune Disease Auto-Antibodies
Anti-dsDNA IgG
ELISA: <201 - Negative | 201-<302 - Equivocal |≥302 - Positive
IU/mL
Anti-Smith IgG
ELFA: <7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Negative
Anti-U1RNP IgG
ELFA: <5 - Negative | 5-10 - Equivocal | >10 - Positive
U/mL
Negative
Anti-RNP70 IgG
ELFA: <7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Negative
Anti-Ro52 IgG
ELFA: <7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Anti-SS-B/La IgG
ELFA: <7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Negative
Anti-Scl-70 IgG
ELFA: <7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Negative
Anti-Centromere Protein B (CENP) IgG
ELFA: <7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Negative
Anti-Jo-1 IgG
ELFA: <7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Negative
Rheumatoid Factor IgM IU/mL
ELFA: <3.5 - Negative | 3.5-5 - Equivocal | >5 - Positive
Rheumatoid Factor IgA
ELFA: <14 - Negative | 14-20 - Equivocal | >20 - Positive
IU/mL
Negative
Anti-CCP IgG
ELFA: <7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Negative
Anti-Ro60 IgG
ELFA: <7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Negative
Anti-Cardiolipin IgM U/mL
Negative ELFA: <10 - Negative | 10-40 - Weak Positive | >40 - Positive
Anti-Cardiolipin IgG
ELFA: <10 - Negative | 10-40 - Weak Positive | >40 - Positive
U/mL
Negative
Anti-β2 Glycoprotein 1 IgM
ELFA: <7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Negative
Anti-β2 Glycoprotein 1 IgG
ELFA: <7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Negative
Anti-Thyroglobulin IgG IU/mL
Negative ELFA: <40 - Negative | 40-60 - Equivocal | >60 - Positive
Anti-Thyroid Peroxidase IgG
ELFA: <25 - Negative | 25-35 - Equivocal | >35 - Positive
IU/mL
Negative
Notes:
22Page of
References
Confirmation by Crithidia luciliae
IFA: Negative
Reference Range
Value Interpretation Reference Range
Value Interpretation Reference Range
Value Interpretation Reference Range
Value Interpretation Reference Range
++
Nuclear Pattern:
>150 STRONG POSITIVE
Negative
Negative
Negative
1:320
Homogeneous
Observed
413.00
1.0
8
125
3.0
1.0
5.0
1.0
1.0
1.0
1.0
2.0
1.0
1.0
3.0
2.0
4.0
3.0
2.0
<12
<4
POSITIVE
POSITIVE
POSITIVE
+
+
+
Rheumatoid Arthritis Auto-Antibodies
Antiphospholipid Syndrome Auto-Antibodies
Thyroid Auto-Antibodies
+
POSITIVE
1) Kalunian K, et al. Measurement of CB-CAPs enhances diagnostic performance in SLE. Arthritis Rheum. 2012 Dec;64(12):4040-7. 2) Wallace D, et al. Systemic lupus erythematosus and primary
fibromyalgia can be distinguished by testing for cell-bound complement activation products. Lupus Sci Med., 2016 Feb;3(1):e000127. 3) Putterman C, et al. CB-CAPS in SLE: comparison with anti-ds DNA
and standard complement measurements. Lupus Sci Med. 2014 Oct;1(1):e000056
Anti-RNA Pol III IgG
ELFA: <7 - Negative | 7-10 - Equivocal | >10 - Positive
U/mL
Negative
10.0
1261 Liberty Way, Vista CA 92081
CLIA# 05D1075048
CAP# 7201051 | NYSDOH PFI# 8369
Laboratory Directors:
Richard Safrin, MD
R. Harper Summers, MD
Provider Relations: 888.452.1522
AVISE® tests are used for clinical purposes, not to be regarded as investigational or for research. Results are not intended to be used as the sole means for clinical diagnosis and patient
management decisions. This test (AVISE CTD) was developed, and performance characteristics were determined by Exagen Inc. as a Laboratory Developed Test (LDT). The Exagen
laboratory is certified under the Clinical Laboratory Amendments of 1988 (CLIA) and accredited by the College of American Pathologists (CAP) as qualified to perform high-complexity
clinical laboratory testing, and FDA approval or clearance is not necessary.
AVISE and the AVISE and Exagen logos are registered trademarks of
Exagen Inc. ©2023 All Rights Reserved
SA1267 (12/22)
AVISE® CTD and AVISE Lupus page 2
Details for ANA (HEp-2) are at the top of the page including any observed nuclear and cytoplasmic patterns
Far left column identies
positive(+) and strong
positive(++) interpretations
for each analyte
Notes provide clarication
and summary statements
Provider Relations: 888.452.1522
Call Provider Relations with any
questions or to arrange for a
clinical consultation
AVISE Interpretation Guide
8
References:
1. Putterman C, Furie R, Ramsey-Goldman R, et al. Cell-bound complement activation products in systemic lupus erythematosus: comparison with anti-double-stranded DNA and standard
complement measurements. Lupus Sci Med. 2014;1(1):e000056. doi:10.1136/lupus-2014-000056
2. Buyon J, Furie R, Putterman C, et al. Reduction in erythrocyte-bound complement activation products and titres of anti-C1q antibodies associate with clinical improvement in systemic
lupus erythematosus. Lupus Sci Med. 2016;3(1):1-8. doi:10.1136/lupus-2016-000165
3. Petri MA, Conklin J, O’Malley T, Dervieux T. Platelet-bound C4d , low C3 and lupus anticoagulant associate with thrombosis in SLE. Lupus Sci Med. Published online 2019:6-11. doi:10.1136/
lupus-2019-000318
4. Yin Y, Wu X, Shan G, Zhang X. Diagnostic value of serum anti-C1q antibodies in patients with lupus nephritis: A meta-analysis. Lupus. 2012;21(10):1088-1097.
doi:10.1177/0961203312451202
5. Hanly JG, Urowitz MB, Su L, Romero-Diaz J. Autoantibodies as biomarkers for the prediction of neuropsychiatric events in systemic lupus erythematosus Ann Rheum Dis. 2011;70(12):2240.
doi:10.1136/ard.2010.148502corr1
6. Russo K, Hoch S, Dima C, Varga J, Teodorescu M. Circulating anticentromere CENP-A and CENP-B antibodies in patients with diuse and limited systemic sclerosis, systemic lupus
erythematosus, and rheumatoid arthritis. J Rheumatol. 2000;27(1):142-148
7. Zampieri S, Ghirardello A, Iaccarino L, Tarricone E, Gambari PF, Doria A. Anti-Jo-1 antibodies. Autoimmunity. 2005;38(1):73-78. doi:10.1080/08916930400022640
8. Homan R.W., Greidinger E.L. (2002) Mixed Connective Tissue Disease. In: Tsokos G.C. (eds) Modern Therapeutics in Rheumatic Diseases. Humana Press, Totowa, NJ. Doi:10.1007/978-1-
59259-239-5_23
9. Basu D, Reveille JD. Anti-scl-70. Autoimmunity. 2005;38(1):65-72. doi:10.1080/08916930400022947
10. Robbins A, et al. Diagnostic Utility of Separate Anti-Ro60 and Anti-Ro52/TRIM21 Antibody Detection in Autoimmune Diseases. Front Immunol. 2019;10:444. doi: 10.3389/
mmun.2019.00444
11. Maes L, et al. Anti-PL/Scl-100 and RNA-Pol III antibodies in scleroderma. Clin Chim Acta. 2010; 411(13-14): 965-71. doi: 10.1016/j.cca.2010.03.018
12. Dalle Vedove C, Simon JC, Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents. J Dtsch Dermatol Ges.
2012;10(12):889-897. doi:10.1111/j.1610-0387.2012.08000.x
13. Rantapää-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis
Rheum. 2003;48(10):2741-2749. doi:10.1002/art.11223
14. Truchetet ME, et al. Association of the presence of anti-carbamylated protein antibodies in early arthritis with a poorer clinical and radiological outcome. Arthritis & Rheumatology.
2017;69(12): 2292-2302. doi: 10.1002/art.40237
15. Monica Galli, Davide Luciani, Guido Bertolini, Tiziano Barbui; Anti–β2-glycoprotein I, antiprothrombin antibodies, and the risk of thrombosis in the antiphospholipid syndrome. Blood.
2003; 102 (8): 2717–2723. doi: 10.1182/blood-2002-11-3334
16. Akhter E, Shums Z, Norman GL, Binder W, Fang H, Petri M. Utility of antiphosphatidylserine/prothrombin and IgA antiphospholipid assays in systemic lupus erythematosus. J Rheumatol.
2013;40(3):282-286. doi:10.3899/jrheum.120084
17. Engler H, Riesen WF, Keller B. Anti-thyroid peroxidase (anti-TPO) antibodies in thyroid diseases, non-thyroidal illness and controls. Clinical validity of a new commercial method for
detection of anti-TPO (thyroid microsomal) autoantibodies. Clin Chim Acta. 1994;225(2):123-136. doi:10.1016/0009-8981(94)90040-x
18. Mahler M, Radice A, Yang W, et al. Clinica Chimica Acta Development and performance evaluation of novel chemiluminescence assays for detection of anti-PR3 and anti-MPO antibodies.
Clin Chim Acta. 2012;413(7-8):719-726. doi:10.1016/j.cca.2012.01.004
19. Damoiseaux J, Csernok E, Rasmussen N, et al. Detection of antineutrophil cytoplasmic antibodies (ANCAs): A multicentre European Vasculitis Study Group (EUVAS) evaluation of the value
of indirect immunouorescence (IIF) versus antigen-specic immunoassays. Ann Rheum Dis. 2017;76(4):647-653. doi:10.1136/annrheumdis-2016-209507
20. Savige J, Gillis D, Benson E, et al. International Consensus Statement on Testing and Reporting of Antineutrophil Cytoplasmic Antibodies (ANCA). Am J Clin Pathol. 999;111(4):507-13. doi:
10.1093/ajcp/111.4.507
21. Wallace ZS, Miloslavsky EM. Management of ANCA associated vasculitis. BMJ. 2020;368(March):1-16. doi:10.1136/bmj.m421
22. Mahler M, Radice A, Sinico RA, et al. Performance evaluation of a novel chemiluminescence assay for detection of anti-GBM antibodies: an international multicenter study. 2012;3(May
2011):24
23. Bossuyt X, Tervaert JW, Arimura Y, et al. Revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheum.
2017;13:683-692. doi: 10.1038/nrrheum.2017.140
24. Merrill J, Petri M, Buyon J, et al. Erythrocyte-bound C4d in combination with complement and autoantibody status for the monitoring of SLE. Lupus Science & Medicine. 2018.
25. Mahler M, Bentow C, O’Malley T, et al. Performance Characteristics of Dierent Anti-Double-Stranded DNA Antibody Assays in the Monitoring of Systemic Lupus Erythematosus. J
Immunol Res. 2017;2017:1720902.
Exagen, AVISE and the Exagen and AVISE logos are registered trademarks of Exagen Inc.
© Copyright 2023 Exagen Inc. All rights reserved. SA1717 (10/23)
www.AviseTest.com | 888.452.1522
