
1
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION
DECISION SUMMARY
A. 510(k) Number:
k090760
B. Purpose for Submission:
New assay
C. Measurand:
Anti-thyroid peroxidase IgG antibodies and anti-thyroglobulin IgG antibodies
D. Type of Test:
Qualitative and quantitative
E. Applicant:
TheraTest Laboratories
F. Proprietary and Established Names:
TheraTest EL - Anti-TPO
TheraTest EL – Anti-Thyroglobulin
G. Regulatory Information:
1. Regulation section:
21 CFR§ 866.5870 – Thyroid Autoantibody Immunological Test System
2. Classification:
Class II
3. Product code:
JZO - System, Test, Thyroid Autoantibodies
4. Panel:
Immunology (82)
H. Intended Use:
1. Intended use(s):
See Indications for Use below.
2. Indication(s) for use:
The TheraTest EL-Anti-TPO™ is an enzyme immunoassay for the qualitative
detection or quantitative determination of IgG class antibodies against thyroid
peroxidase (TPO) in human serum. The TheraTest EL-Anti-TPO™ assay is
intended for use as an aid in the diagnosis of autoimmune thyroid disorders, in
conjunction with other clinical and laboratory findings.
The TheraTest EL-Anti-Thyroglobulin™ is an enzyme immunoassay for the
qualitative detection or quantitative determination of IgG class antibodies against
thyroglobulin in human serum. The TheraTest EL-Anti-Thyroglobulin™ assay is
intended for use as an aid in the diagnosis of autoimmune thyroid disorders, in
conjunction with other clinical and laboratory findings.
3. Special conditions for use statement(s):
For prescription use only.
4. Special instrument requirements:
Spectrophotometric microplate reader with single (450 nm) or dual (450 nm, 620-
2
690 nm reference) wavelength and ELISA plate washer (optional).
I. Device Description:
Each device contains the following: two 96-well ELISA plates with breakaway strips
coated with native human thyroid peroxidase (TPO) or native human thyroglobulin
(Tg); a plate frame; assay controls (human serum with or without IgG antibodies
against TPO or Tg; ready to use calibrators; goat anti-human IgG (Fcγ specific)
horseradish peroxidase conjugate; TMB chromogen; wash buffer (10X) and stop
solution.
J. Substantial Equivalence Information:
1. Predicate device name(s):
Architect Anti-Tg
Architect Anti-TPO
2. Predicate K number(s):
k052308
k052407
3. Comparison with predicate:
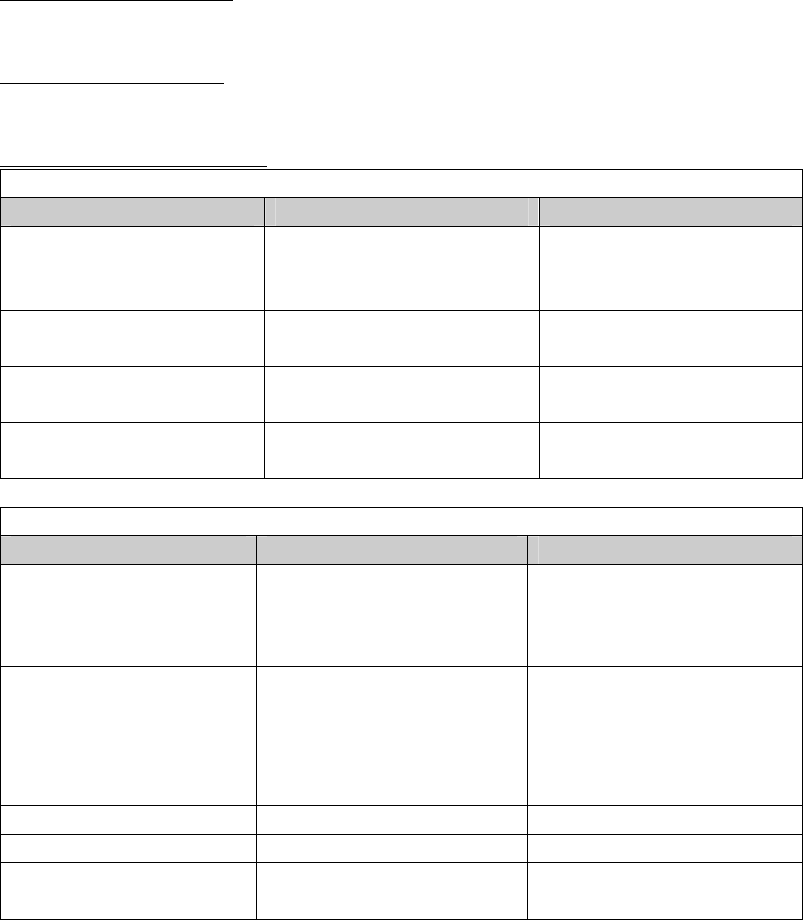
Similarities
Item Device Predicate
Intended use aid in detection of
autoimmune thyroid
disease
same
Assay Range TPO: LoD – 1000 IU/mL
Tg: LoD – 2000 IU/mL
same
Analyte anti-TPO antibodies
anti-Tg antibodies
same
Calibration MRC 65/093 for Tg Ab,
MRC 66/387 for TPO Ab
same
Differences
Item Device Predicate
Conjugate goat anti-human IgG (Fcγ
specific) horseradish
peroxidase
Anti-human IgG (mouse
monoclonal) acridinium
labeled conjugate in MES
buffer with protein (bovine)
Capture Antigen human thyroglobulin- or
TPO- coated microwells
Human thyroglobulin- or
TPO-coated
microparticles in MES
buffer with protein (goat)
stabilizer.
Detection Method colorimetric chemiluminescence
Sample Matrix serum only serum and plasma
Assay Format quantitative and
qualitative manual
quantitative automated
K. Standard/Guidance Document Referenced (if applicable):
None referenced.
3
L. Test Principle:
The TheraTest EL-TPO and the TheraTest EL-Thyroglobulin are solid phase enzyme
immonoassays in a 96-well plate format for the measurement of antibodies against
tissue TPO or thyroglobulin. Wells are incubated with diluted samples, calibrators,
and positive and negative controls. During the incubation, anti-TPO or anti-TG
antibodies, if present, bind to the solid phase antigen. The wells are washed, and
isotype-specific horseradish peroxidase labeled anti-human immunoglobulin antibody
(enzyme conjugate) is added. After incubating the wells with the enzyme conjugate,
unbound labeled antibody is removed by washing. A chromogenic substrate solution
is added to the wells, and the presence of antibodies to TPO or Tg is detected by a
color change. The intensity of the color is proportional to the amount of the bound
antibody and is read by an ELISA reader. The absorbance value in the blank well
(incubated with specimen diluent) is subtracted from the values obtained with
samples, calibrators and controls.
In the quantitative mode, the absorbance of the sample is converted to a relative value
based on the standard curve generated by the calibrators. In the qualitative mode, the
result is based on a ratio of the sample to the cut-off calibrator. If the specimen's
measured absorbance value exceeds that of the highest Calibrator, the result is
reported as >IU/mL (of the highest Calibrator). If exact determination is required, the
specimen should be pre-diluted in the provided Specimen Diluent before assaying,
and the results should be calculated by taking the dilution factor into account.
M. Performance Characteristics (if/when applicable):
1. Analytical performance:
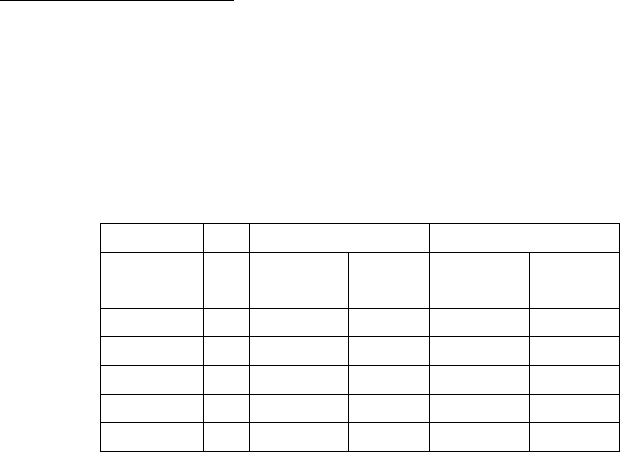
a. Precision/Reproducibility:
Precision: For both assays, two specimens were tested 20 times within the
same assay (within-run precision) and 20 different times in one or two runs
per day (between-run precision). Three additional samples were tested in
triplicate in 10 runs, one or two runs per day. The results are presented in the
following tables:
Imprecision of the EL-Anti-TPO Assay
Within-run Between-run
Mean
(IU/mL)
n Std Dev % CV Std Dev % CV
4.0 30 0.3 7.3 % 0.45 11.3 %
12.5 20 0.6 4.9 % 0.9 8.1 %
22.1 30 0.5 2.7 % 1.7 7.8 %
243.9 20 9.6 3.9 % 22.2 10.9 %
697.9 30 51.7 7.4 % 89.9 12.9 %
4
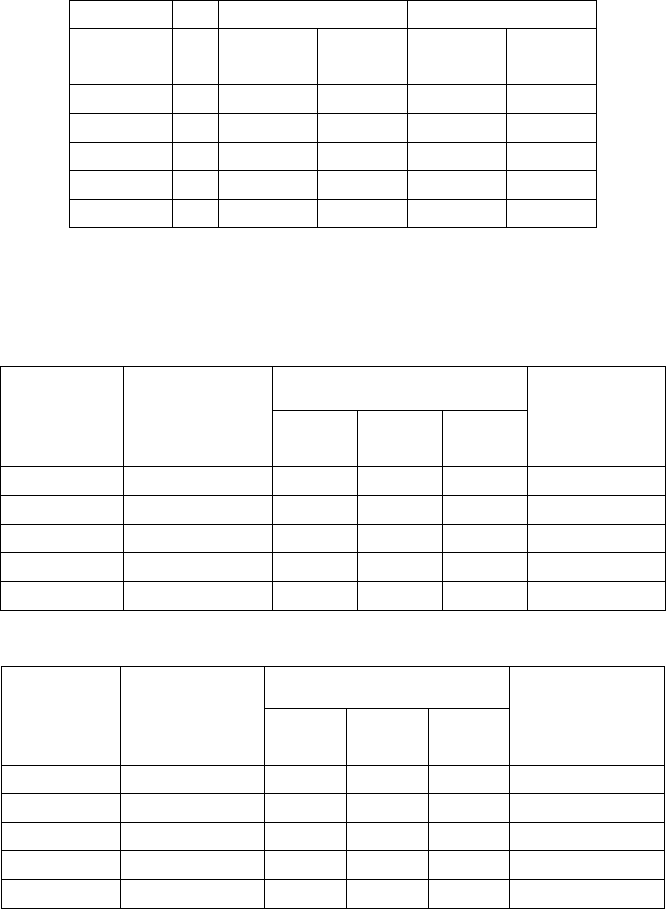
Imprecision of the EL-Anti-Thyroglobulin Assay
Within-run Between-run
Mean
(IU/mL)
n Std Dev % CV Std Dev % CV
19.0 30 2.1 10.7 % 2.2 11.5 %
41.5 20 1.0 2.4 % 4.3 10.0 %
67.4 30 1.7 2.5 % 6.8 10.1 %
607.3 20 18.9 3.1 % 51.6 9.6 %
1257 30 67.1 5.3 % 83.0 6.6 %
Reproducibility: The reproducibility of both assays was evaluated by testing
clinical samples across the claimed assay range 20 or 30 times each:
Reproducibility of the EL Anti-Thyroglobulin Assay
Test result: Sample
Value*
(IU/mL)
Expected
Qualitative
Result:
Neg Equi-
vocal
Pos
% Expected
Result
19.0 negative 30 0 0 100%
43.4 equivocal 0 18 2 90%
67.4 positive 0 0 30 100%
535.1 positive 0 0 20 100%
1257 positive 0 0 30 100%
Reproducibility of the EL Anti-TPO Assay
Test result: Sample
Value*
(IU/mL)
Expected
Qualitative
Result:
Neg Equi-
vocal
Pos
% Expected
Result
4.0 negative 30 0 30 100%
11.3 equivocal 0 17 3 85%
22.1 positive 0 0 30 100%
203.4 positive 0 0 20 100%
697.9 positive 0 0 30 100%
* Sample Value determined with the quantitative protocol.
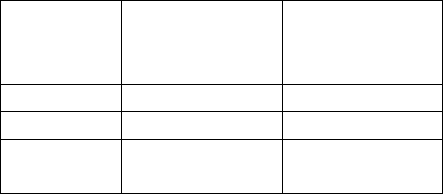
b. Linearity/assay reportable range:
The claimed range of the EL Anti-TPO Assay is 0.4 – 1000 IU/mL; the
claimed range of the EL Anti-Thyroglobulin Assay is 3.0 – 2000 IU/mL. To
test whether the assays were linear over their claimed range, six samples from
different parts of the assay range were diluted between 6 and 10 times. The
dilutions were tested with the appropriate TheraTest assay. The measured
values were pooled and plotted against the expected values and linear regression
analysis was performed:

5
TheraTest EL Anti-Thyroglobulin
y = 1.004x + 25.75
R
2
= 0.996
0.0
200.0
400.0
600.0
800.0
1000.0
1200.0
1400.0
1600.0
1800.0
0.0 500.0 1000.0 1500.0 2000.0
Target IU/mL
Obtained IU/mL
TheraTest EL Anti-TPO
y = 0.944x - 9.426
R
2
= 0.991
0.0
200.0
400.0
600.0
800.0
1000.0
1200.0
0.0 200.0 400.0 600.0 800.0 1000.0 1200.0
Target IU/mL
Obtained IU/mL
6
The sponsor presented a study that supported the claim that there is no hook
effect up to 17,000 IU/mL in the EL-Anti-TPO assay, and up to 16,000 IU/mL
in the EL-Anti-Thyroglobulin™ assay.
c. Traceability, Stability, Expected values (controls, calibrators, or methods):
Assay calibrators are human serum specimens containing anti-Tg or anti-TPO
antibodies. The concentration is determined for every new lot; every new lot
of calibrators is assigned IU/mL values by validating them against the
International Reference Preparations, MRC 65/093 for Tg Ab, MRC 66/387
for TPO Ab.
An accelerated stability study supports a claim of unopened kit stability of one
year; real-time stability studies are underway. Opened kit stability is one year.
Studies of serum sample stability show that the stability of the antibodies in
serum for one week at 4 – 8 °C.
d. Detection limit:
The Limit of Blank (LoB) was calculated from reading the zero calibrator 20
times. The Limit of Detection (LoD) was calculated as the LoB
+ 1.65*SD (low
concentration specimen). Here the low concentration specimen was the negative
control. The functional sensitivity of the assays was defined as the lowest
concentration that could be quantitatively determined with acceptable precision
(< 20%). Two samples with low levels of analyte were tested in triplicates in six
runs and the coefficient of variation was calculated. The functional sensitivity
claims in the table below are supported by the provided data:
EL-Anti-TPO
assay (IU/mL)
EL-Anti-
Thyroglobulin
assay (IU/mL)
LoB 0.05 0.44
LoD 0.40 3.0
Functional
Sensitivity
1.3 8.2
e. Analytical specificity:
Serum samples containing known levels of other autoantibodies [anti-dsDNA,
anti-myeloperoxidase (MPO) and anti-cyclic citrullinated peptide (CCP)
antibodies] were added to anti-TPO and anti-thyroglobulin positive serum
specimens and the recovery (%) of the anti-TPO and anti-thyroglobulin
antibody concentrations were calculated. There was no significant decrease in
recovery. Samples positive for anti-dsDNA, anti- MPO and anti-CCP
antibodies were tested with the anti-TPO and anti-thyroglobulin assays but
showed no activity.
Cross-reactivity between anti-thyroglobulin and anti-TPO antibodies was
examined by studying the inhibition of two serum specimens containing both
anti-TPO and anti-thyroglobulin antibodies. These samples were incubated
with 10 µg TPO antigen, or 10 µg thyroglobulin antigen, or the same volume
of Specimen Diluent, and then assayed in duplicate with the EL-Anti-TPO™

7
and EL-Anti-Thyroglobulin™ kits. In sample 1, incubation with TPO antigen
resulted in 67.5 % inhibition of autoantibody binding in the anti-TPO test but
minimal inhibition (15.7 %) of the anti-thyroglobulin test. Incubation with
thyroglobulin antigen resulted in 88.7% inhibition of autoantibody binding in
the anti-thyroglobulin test, but no inhibition (1.5 %) in the anti-TPO test. In
sample 2, incubation with TPO antigen resulted in 97.3 % inhibition of
autoantibody binding in the anti-TPO test but no inhibition (0.6 %) in the anti-
thyroglobulin test. Incubation with thyroglobulin antigen resulted in 80.8 %
inhibition of autoantibody binding in the anti-thyroglobulin test, but no
inhibition (-3.7 %) in the anti-TPO test.
The sponsor did not evaluate the effect of endogenous or exogenous
substances on the performance of the assays. The package insert contains a
caution not to use hemolyzed, lipemic, or icteric samples.
f. Assay cut-off:
See Expected Values/Reference Range section below.
2. Comparison studies:
a. Method comparison with predicate device:
One hundred nine serum samples that had been sent to a reference laboratory
for thyroid testing were acquired, stored frozen, and then tested by the
sponsor. The samples were not individually identifiable and were not
accompanied with clinical or demographic data. Agreement between the
proposed assays and the predicate assays is described below:
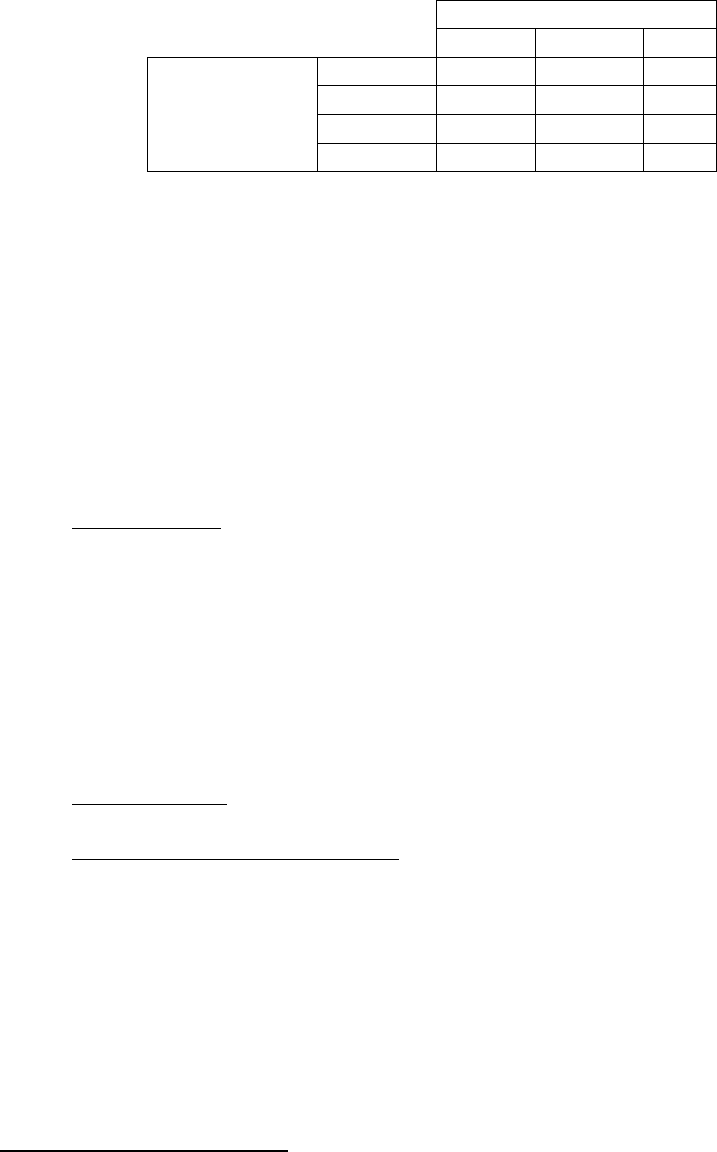
Agreement between Predicate and EL-Anti-TPO Assays:
Predicate Assay
Positive Negative Total
Positive 62 0 62
Negative 4 41 45
Equivocal* 2 0 2
EL-Anti-TPO
Total 68 41 109
Percent Agreement with equivocal results considered negative in calculations:
Positive Agreement: (62/68)*100 = 91.2% (95% CI: 81.8% to 96.7%)
Negative Agreement: (41/41)*100 = 100.0% (95% CI: 91.4% to 100%)
Total Agreement: (103/109)*100 = 94.5% (95% CI: 88.4% to 98.0%)
Percent Agreement with equivocal results considered positive in calculations:
Positive Agreement: (64/68)*100 = 94.1% (95% CI: 85.6% to 98.4%)
Negative Agreement: (41/41)*100 = 100.0% (95% CI: 91.4% to 100%)
Total Agreement: (105/109)*100 = 96.3% (95% CI: 90.9% to 99.0%)
8
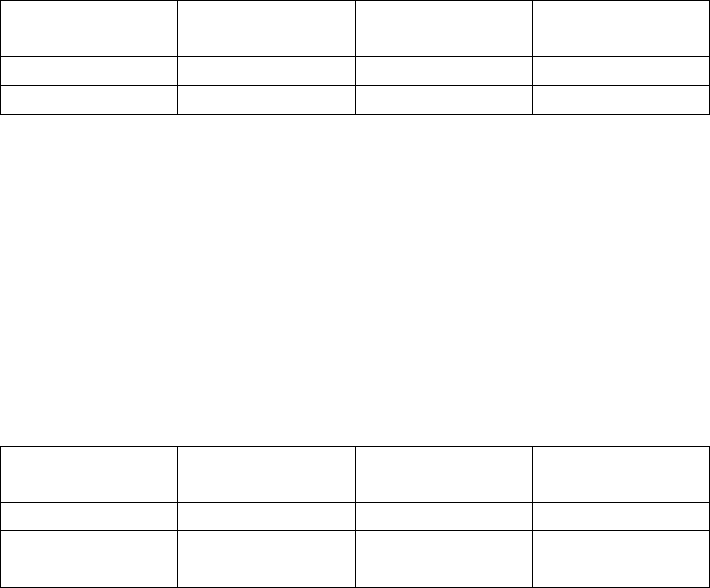
Agreement between Predicate and EL-Anti-Thyroglobulin Assays:
Predicate Assay
Positive Negative Total
Positive 77 1 78
Negative 1 29 30
Equivocal 1 0 1
EL-Anti-
Thyroglobulin
Total 79 30 109
Percent Agreement with equivocal results considered negative in calculations:
Positive Agreement: (77/79)*100 = 97.5% (95% CI: 91.2% to 99.7%)
Negative Agreement: (29/30)*100 = 96.7% (95% CI: 82.8% to 99.9%)
Total Agreement: (106/10)*100 = 97.2% (95% CI: 92.2% to 99.4%)
Percent Agreement with equivocal results considered positive in calculations:
Positive Agreement: (78/79)*100 = 98.7% (95% CI: 93.1% to 100%)
Negative Agreement: (29/30)*100 = 96.7% (95% CI: 82.8% to 99.9%)
Total Agreement: (107/10)*100 = 98.2% (95% CI: 93.5% to 99.8%)
b. Matrix comparison:
This assay is only indicated for serum samples.
3. Clinical studies:
a. Clinical Sensitivity:
The sponsor makes no claim of clinical sensitivity. Claims of clinical
sensitivity should be supported by comparing test performance to known
clinical diagnoses.
b. Clinical specificity:
The sponsor makes no claim of clinical specificity. Claims of clinical
specificity should be supported by comparing test performance to known
clinical diagnoses.
c. Other clinical supportive data (when a. and b. are not applicable):
4. Clinical cut-off:
Not applicable to these assays.
5. Expected values/Reference range:
The National Academy of Clinical Biochemistry
1
has suggested reference
intervals for thyroid antibody tests should be established on controls selected by a
process that would be least likely to include subjects with a predisposition to
autoimmune thyroid disease. Based on this recommendation, 108 male healthy
blood bank donors with no history of thyroid disease or non-thyroid autoimmune
disease were selected as a control population. The median age was of 36 years
(range was 16 - 72 years) and predominately Caucasian (70%). Pediatric controls
were not included. The TPO antibody and thyroglobulin antibody concentrations
in the serum samples of the control subjects were measured with the EL-Anti-
1
Demers LM, Spencer CA. Laboratory Medicine Practice Guidelines; Laboratory
Support for the Diagnosis and Monitoring of Thyroid Disease. Eds. LM Demers
and CA Spencer. Thyroid Vol. 13:3 2003.
9
TPO and the EL-Anti-Thyroglobulin assays. The results were ranked in
descending order, and the cut-off values were obtained by percentile ranking of
the values. The results were calculated according to both the quantitative and the
qualitative methods.
Quantitative method:
According to the quantitative method, the 97.5
th
percentile concentration was 13.1
IU/mL for the anti-TPO assay and 40.8 IU/mL for the anti-thyroglobulin assay.
To reflect the fact that the healthy and the diseased populations have overlapping
autoantibody values, the sponsor introduced equivocal zones around these
concentrations. The resulting reference ranges are:
Negative
(IU/mL)
Equivocal
range (IU/mL)
Positive range
(IU/mL)
Anti-TPO ≤ 10 IU/mL 11-15 ≥ 16
Anti-Tg ≤ 35 IU/mL 36-50 ≥ 51
For the TPO assay, 96.1% of the control population values were in the negative
reference range while 2.9% were positive and 0.9% was in the equivocal range.
For the thyroglobulin assay, 96.1% of the control population values were in the
reference range, 1.9% were positive and 1.9% were in the equivocal range.
Qualitative method:
Results were also calculated according to the qualitative protocol and were
expressed in U/mL. Reference ranges and equivocal zones were determined the
same way as described above for the quantitative method. The following
reference ranges and equivocal zones were established:
Negative
(U/mL)
Equivocal
range (U/mL)
Positive range
(U/mL)
Anti-TPO ≤ 5 6 - 8 ≥ 9
Anti-
Thyroglobulin
≤ 20 21 -30 ≥ 31
Using the qualitative method determination, 96.1% of the control population had
TPO values in the negative reference range, 2.9% were positive and 0.9% was in
the equivocal range. For the thyroglobulin assay, 96.1% of the control population
had values in the reference range, 1.9% were positive and 1.9% were in the
equivocal range.
N. Proposed Labeling:
The labeling is sufficient and it satisfies the requirements of 21 CFR Part 809.10.
O. Conclusion:
The submitted information in this premarket notification is complete and supports a
substantial equivalence decision.
