Laboratory Testing Reference Guide
an Alere company.


3
REFERENCE GUIDE // Result reporting
REFERENCE GUIDE // Table of contents
TABLE OF contents
LABORATORY TESTING SERVICES 5
Drugs of Abuse Testing – Urine ...................................6-8
Drugs of Abuse Testing – Oral Fluid...............................9-10
Esoteric/Specialty Testing ........................................11
SPECIMEN COLLECTION 13
Collection: Site Preparation .......................................14
Collection: Urine/Oral Fluid Collection Protocol .....................15-16
Collection: Specimen Validity................................... 17-18
Collection: Problematic Situations ...............................19-20
SPECIMEN LABELING & SHIPPING 23
Option 1: ToxAccess
™
Labeling Guide ............................24-25
Option 2: Urine Test Request Labeling Guide ......................26-27
Option 3: Oral Fluid Test Request Labeling Guide ...................28-29
Packaging Protocol: Shipping to Lab.............................30-31
RESULT REPORTING & COLLECTION MANAGEMENT 33
ToxAccess
™
: Web Result Reporting & Collection Management ........... 34
Standard Result Reporting ...................................... 35
DRUG INFORMATION 37
Alcohol ..................................................... 38
Amphetamines ............................................... 39
Barbiturates.................................................. 40
Benzodiazepines ...............................................41
Buprenorphine ............................................... 42
Cocaine..................................................... 43
Designer Stimulants ........................................... 44
Diuretics .................................................... 45
Fentanyl..................................................... 46
Gamma-Hydroxybutyric Acid (GHB) ............................... 47
Marijuana (cannabinoids)........................................ 48
Methadone .................................................. 49
Opiates ..................................................... 50
Oxycodone . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .51
Phencyclidine (PCP) ........................................... 52
Steroids..................................................... 53
Stimulants ................................................... 54
Synthetic Cannabinoids......................................... 55
Urine Creatinine............................................... 56
CONTACT INFORMATION 58


5
Laboratory services
Redwood Toxicology Laboratory (RTL) performs drug and alcohol testing in accordance
with strict forensic standards and scientically accepted methods. Testing is performed by
a highly educated, experienced staff using state-of-the-art equipment under the scrutiny of
state and federal agencies. We’ll nd out.
Drugs of Abuse Urine Testing — RTL processes over 85,000 urine specimens each
week for thousands of clients.
Drugs of Abuse Oral Fluid Testing — Oral uid testing is gaining popularity with
many programs that require easy, gender-neutral specimen collection combined with
the accuracy of lab testing.
Esoteric/Specialty Testing — RTL also offers a wide range of specialized tests includ-
ing: EtG/EtS Alcohol testing, Synthetic Cannabinoid testing, Designer Stimulants testing,
Comprehensive drug testing, GHB testing, Fentanyl testing and hCG (pregnancy) testing
and more.
REFERENCE GUIDE // Laboratory services

laboratory services
6
OVERVIEW
The following is an explanation of Redwood Toxicology
Laboratory’s (RTL) urine screening and conrmation
procedures/cutoff levels. The routine cutoff levels listed
may periodically change. Note: some cutoff levels may
differ for your agency. The analytical methods used by
RTL are scientically accepted and approved by the
U.S. Department of Health and Human Services.
URINE SCREENING METHODOLOGY
In order to determine if a urine specimen is negative or positive
for drugs of abuse, all specimens are initially screened by an
enzyme immunoassay (EIA) procedure. Specimens that yield
an EIA response below the specied cutoff are reported as not
detected. Specimens that show an EIA response at or above
the specied cutoff are considered “presumptive positive” for a
particular drug or drug class. Based on your agency’s account,
presumptive positive specimens may be conrmed by a second
method prior to reporting positive results. (See “urine conrma-
tion methodology”).
Screening Cutoff Levels by Method
DRUG METHOD CUTOFF
Amphetamines
(Amphetamine/Methamphetamine)
EIA 500/1000 ng/mL
Barbiturates EIA 200 ng/mL
Benzodiazepines EIA 200 ng/mL
Buprenorphine EIA 5 ng/mL
Cocaine Metabolite (Benzoylecgonine) EIA 150/300 ng/mL
Cotinine EIA 250 ng/mL
Dextromethorphan (DXM) ELISA 125 ng/mL
Ecstasy (MDMA) EIA 500 ng/mL
Ethanol EA 0.04 gm/dL
EtG EIA 100/500 ng/mL
LSD (Lysergic Acid Diethylamide) ELISA 100 pg/mL
6-MAM (Heroin Metabolite) EIA 10 ng/mL
Methadone EIA 150 ng/mL
Methadone Metabolite EIA 150 ng/mL
Methaqualone EIA 300 ng/mL
Meprobamate EIA 100 ng/mL
Opiates (Morphine and Codeine) EIA 300 ng/mL
Oxycodone EIA 300 ng/mL
Phencyclidine EIA 25 ng/mL
Propoxyphene EIA 300 ng/mL
Tramadol EIA 200 ng/mL
THC (Cannabinoids) EIA 20/50 ng/mL
1
Drugs of Abuse Testing — Urine
Routine laboratory urine testing services
Cutoff levels u pdated p eriodical ly
Cutoff levels updated periodically
URINE CONFIRMATION METHODOLOGY
Analytical methods of conrmation include, gas chromatography (GC), gas chromatog-
raphy/mass spectrometry (GC/MS) or liquid chromatography/mass spectrometry/mass
spectrometry (LC/MS/MS). The subsequent conrmatory procedures are performed on
a second independent portion of the original urine specimen.
Confirmation Cutoff Levels by Method
DRUG GC/MS LC/MS/MS
Alcohol (ethanol) .02 gm/dL
(GC-FID)
2
Amphetamines
- Amphetamine / Methamphetamine /
MDA / MDEA / MDMA
250 ng/mL
Barbiturates 200 ng/mL
Benzodiazepines 50 ng/mL
Buprenorphine/Norbuprenorphine 0.5 ng/mL
Cocaine
1
50 ng/mL
Dextromethorphan (DXM) 50 ng/mL
EtG 100 ng/mL
EtS 25 ng/mL
Fentanyl 5 ng/mL
GHB 10 mcg/mL
Marijuana Metabolite (THC-COOH) 5 ng/mL
Methadone / Methadone Metabolite (EDDP) 100 ng/mL
Opiates
- Total Morphine / Codeine 100 ng/mL
- 6-Monoacetylmorphine 5 ng/mL
- Hydrocodone / Hydromorphone 100 ng/mL
- Oxycodone / Oxymorphone / Noroxycodone 50 ng/mL
Phencyclidine (PCP) 10 ng/mL
Propoxyphene 200 ng/mL
Tricyclic Antidepressants 25 ng/mL
Seditive / Hypnotic Agents
- Carisoprodol 100 ng/mL
- Meprobamate 200 ng/mL
- Zolpidem 1 ng/mL
- Carboxyzolpidem 10 ng/mL
1. Agency has the ability to choose cutoff levels indicated.
2. Test performed by Gas Chromatography Flame Ionization Detection (GC/FID)

REFERENCE GUIDE // Laboratory services
7
Drug Class Trade Name Street Names Detection Time
1
DEA†
ANALGESICS (Synthetic)
Meperidine Demerol
®
Demmies, pain killer 1-3 days II
Methadone Methodose
®
, Dolophine
®
Dollies, meth 1-7 days II
Pentazocine Talwin
®
T’s 1-3 days III
Propoxypene Darvocet
®
, Darvon
®
Pain killer 1-7 days IV
CANNABINOIDS
Cannabinoids Marinol
®
, marijuana, pot, reefer Mary Jane, Grass, Pot, Smoke, Weed See below
2
I
HALLUCINOGENS
Lysergic acid diethylamide None LSD, Acid 1.5-5 Days I
Methylenedioxyamphetamine None MDA, Love Drug 1-3 days I
Methylenedioxymethamphetamine (MDMA) Ecstasy MDMA, Adam, Ecstasy, X 1-3 days I
Phencyclidine PCP PCP, Angel Dust 2-30 days II
DEPRESSANTS/SEDATIVES/HYPNOTICS
Barbiturates
Amobarbital Amytal
®
Yellow jackets, angels, downers 3-10 days III
Butabarbital Butisol
®
, Butibel
®
Yellow jackets, angels, downers 1 day–3 weeks III
Butalbital Fiorinal
®
, Fioricet
®
Yellow jackets, angels, schoolboys 3-10 Days III
Pentobarbital Nembutal
®
Yellow jackets, angels, phennies 3-10 Days III
Phenobarbital Belladonna
®
, Luminal
®
Yellow jackets, phennies, goofballs 1 day–4 weeks IV
Secobarbital Seconal
®
Yellow jackets, Barbs, Reds 3-10 days III
Benzodiazepines
Alprazolam Xanax
®
, Niravam
®
, Xanor
®
Downs, Nerve Pills, Tranks 2-8 days IV
Chlordiazepoxide Librium
®
, Angirex
®
, Elenium
®
“ 1-7 Days IV
Clonazepam Klonopin
®
“ 3-14 days IV
Clorazepate Tranxene
®
, Novo-Clopate
®
“ 1-7 Days IV
Diazepam Valium
®
“ 3-14 days IV
Flurazepam Dalmane
®
, Dalmadorm
®
“ 1-3 days IV
Lorazepam Ativan
®
Lorax
®
, Emotavil
®
“ 2-5 days IV
Midazolam Versed, Dormicum, Hypnovel
®
“ 1-3 days IV
Oxazepam Murelax
®
, Serax
®
, Serepax
®
“ 1-3 days, 4-6 weeks Chronic use
3
IV
Methaqualone Quaalude Ludes IV
Tricyclic Antidepressants
Amitriptyline Elavil
®
, Endep
®
None 2-14 days
Desipramine Norpramin
®
“
3-15 days
Doxepin Sinaquan
®
, Adapin
®
“ 2-8 days
Imipramine Tofranil
®
, Tofranil-PM “ 1-6 days
Maprotiline Ludiomil
®
, Deprilept
®
, Psymion
®
“ 7-10 days
Nortriptyline Pamelor
®
, Aventyl
®
“ 4-21+ days
Alcohol
Ethanol N/A Booze
Alcohol: 1-2 days
blood alcohol decreases ~.02 gm% per hour
OPIATES/ANALGESICS (Semi-Synthetic)
Codeine Tylenol
®
#3 Schoolboy 1-3 days II and III
Diacetylmorphine Heroin Horse, Smack, “H”, Speedball (w/ Cocaine) 6-MAM 6-8 hours 1-3 days II and III
Hydrocodone Hycodan
®
, Vicodin
®
, Lortab
®
Hydros, dones, vics, itchies 1-3 days II
Hydromorphone Dilaudid
®
, Hymorphan
®
Juice, dillies, M2s, hospital heroin 1-3 days II
Morphine Roxanol
®
, Avinza
®
, Kadian
®
“M”, morph 1-3 days II
Oxycodone Oxycontin
®
, Percodan
®
, Percocet
®
Oxy-cofns, killers, percs 1-3 days II
Oxymorphone Opana ER
®
, Opana IR
®
, Numorphan
®
Blues, nu-blues, biscuits, blue heaven 1-3 days II
STIMULANTS
Amphetamine Adderall
®
, Benzedrine
®
, Dexedrine
®
Aimies, back dex, bennies 2-4+ days II
Cocaine None Coke, rock, crack, snow, blow, toot 1-7 days II
Methamphetamine Desoxyn
®
, Methadrine
®
Crystal meth, crystal, meth 1-4+ days II
1) Average detection times
2) Cannabinoids Detection Time
Single use: 3 days 1-3 Days
Moderate use: 5-7 days 5-7 Days
Daily use: 10-15 days 10-15+ Days
Long term: >30 days 30+ Days
Oral Ingestion 1-5 Days
3) Chronic use over period of months or years; 4-6 weeks
I Illicit drugs with no medical use; high potential for abuse
II Prescription drugs with high potential for abuse and physical dependency
III Drugs with less abuse potential than schedule II; have moderate to low physical dependency,
but may have high psychological dependence
IV Prolonged use of these drugs may lead to limited physical or psychological dependency; lower
abuse potential than schedule III
† Explanation of Drug Enforcement Agency (DEA) Classification
Urine Drug Testing: Classification Table & Detection Time
Cutoff levels updated periodically

laboratory services
8
FREQUENTLY ASKED QUESTIONS
Who should consider urine drug testing?
Redwood Toxicology Laboratory’s (RTL) Urine Drug Testing is
recommended for a large variety of arenas, including the following:
Federal & State Corrections, Clinics & Hospitals, Methadone Clinics,
Federal, State & County Probation, Counseling Centers, Physician
Ofces, Federal Halfway Houses, Drug Courts, Pre-employment,
Behavioral Health, Jails & Detention Centers, Rehabilitation &
Treatment, Child & Family Services, Mental Health and Schools
& Universities.
How do I collect the urine and send in a specimen?
Refer to page 13-20 for specimen collection protocols. Instructions
for use are also included with your rst order of lab supplies. Please
read these instructions carefully. RTL offers telephonic training and
instructional materials.
How long can the urine specimen be stored before testing?
Specimens can be stored at room temperature up to 7 days. Urine
specimens should be refrigerated if testing is delayed. However, it is
strongly recommend that the sample be tested as soon as possible
after collection.
What testing methodology does RTL use
to perform initial drug screening?
RTL screens urine specimens by enzyme immunoassay (EIA). An
immunoassay is a test that uses antibodies to detect the presence
of drugs and other substances in urine. The initial screening process
does not measure the specic amount of drug present in urine
samples. It provides either a positive or negative result, indicating
the presence or absence of detectable drug metabolites above a
specic cutoff level.
What testing methodology does RTL use
for conrmations?
Conrmations are available by gas chromatography (GC), gas
chromatography/mass spectrometry (GC/MS) and liquid chromatog-
raphy/mass spectrometry/mass spectrometry (LC/MS/MS). Based
on your agency’s account settings, specimens may be conrmed by
one or more of the aforementioned methods. GC/MS and LC/MS/
MS provides identication of the molecule(s) based on characteristic
fragmentation patterns at specic retention times.
Why are screening and conrmation cutoff
levels different?
Screening and conrmation testing are performed using different
methodologies that necessitate different cutoff levels. The cutoff
levels of an immunoassay screen are typically higher than those of
a more sensitive GC/MS or LC/MS/MS conrm test, because they
screen for a larger group of parent compounds, metabolites and
other structurally similar compounds.
If an immunoassay test detects a drug (above the screening cutoff
level) the presumptive positive specimen may be sent to GC/MS
or LC/MS/MS conrmation testing. Many times, these individual
compounds are present in concentrations much lower than the total
immunoassay response, thus resulting in the cutoff levels being
lower for the GC/MS or LC/MS/MS test.
What is the importance of checking the urine
temperature strip on the collection cup?
Under normal situations fresh urine will display a temperature
between 90 and 100 degrees Fahrenheit on the temperature strip,
if read within 4 minutes of the collection. Should the temperature
strip not register, the specimen should be immediately re-checked
using a new cup (or strip) and the results recorded on the requisi-
tion. Specimens with a temperature out of range may indicate a
substituted or adulterated sample.
How long does it take for results?
Urine screening results are typically available within 24 hours of
receipt of the specimen, while oral uid screen results take 24-48
hours. Presumptive positive specimens are usually conrmed
(unless they are “Screen Only”) within 24-72 hours depending on
the method. Conrmation of specimens that are presumptive posi-
tive by instant/on-site devices take a minimum of 48 hours.
What does ng/mL mean?
Drug testing cutoff levels are usually expressed in the units of mea-
sure ng/mL (nanograms per milliliter). A quantitative positive GC/MS
or LC/MS/MS result is commonly expressed in ng/mL.
Urine drug testing continued

REFERENCE GUIDE // Laboratory services
9
OVERVIEW
Oral uid testing is gaining popularity with many programs
that require convenient, gender-neutral specimen collec-
tion combined with the accuracy of lab testing. Redwood
Toxicology Laboratory (RTL) provides an easy and afford-
able lab-based testing solution for the detection of drugs
of abuse in oral uid. RTL’s oral uid testing utilizes a
collection device that has a volume adequacy indica-
tor. This indicator ensures that sufcient saliva (1 mL) is
collected to prevent possible false negative results due
to insufcient sample size, and to provide a meaningful
quantitative result.
The collection device also allows drug concentrations
to be reported per mL of oral uid (GC/MS and LC/MS/
MS conrmations only). One (1) mL of oral uid combined
with three (3) mL of buffering agent provides four (4) mL
of specimen, allowing a sufcient amount of sample for
screening and conrmation procedures.
The following is an explanation of RTL’s saliva screening
and conrmation procedures/cutoff levels. The routine
cutoff levels listed below may periodically change. Note:
some cutoff levels may differ for your agency.
SALIVA SCREENING METHODOLOGY
Specimens collected with the oral uid collection device are sent to
Redwood Toxicology Laboratory for screening by Enzyme Immuno-
Assay (EIA) or Enzyme-Linked Immunosorbent Assay (ELISA).
Screening Cutoff Levels by EIA
DRUG EIA
Alcohol .025 g/dL
Amphetamine 50 ng/mL
Barbiturates
1
50 ng/mL
Benzodiazepines 20 ng/mL
Cocaine
2
20 ng/mL
Methadone 50 ng/mL
Methamphetamine 50 ng/mL
Opiates (codeine, hydrocodone, hydromorphone,
morphine, 6-monoacetylmorphine)
40 ng/mL
Oxycodone 40 ng/mL
Phencyclidine 10 ng/mL
THC (r-9-THC) 4 ng/mL
SALIVA CONFIRMATION METHODOLOGY
Positive screens are conrmed by gas chromatography/mass
spectrometry (GC/MS) or liquid chromatography-tandem mass
spectrometry (LC/MS/MS).
Confirmation Cutoff Levels by Method
DRUG METHODOLOGY CUTOFF
Alcohol GC-FID .025 g/dL
Amphetamine GC/MS 15 ng/mL
Barbiturates GC/MS 20 ng/mL
Benzodiazepines
LC/MS/MS 0.5 ng/mL
Buprenorphine LC/MS/MS 1 ng/mL
Norbuprenorphine LC/MS/MS 5 ng/mL
Cocaine
2
GC/MS 8 ng/mL
Methadone GC/MS 10 ng/mL
Methamphetamine GC/MS 15 ng/mL
Opiates
- Codeine, Hydrocodone,
Hydromorphone, Morphine
GC/MS
20 ng/mL
- 6-monoacetylmorphine
GC/MS
4 ng/mL
Oxycodone GC/MS 20 ng/mL
Phencyclidine GC/MS 5 ng/mL
THC (r-9-THC) GC/MS 1 ng/mL
Oral Fluid Detection Times
DRUG Detection Time
3
Alcohol
After absorption (~1 hour) blood
alcohol decreases ~.02 gm%/hour
Amphetamine From minutes up to 48 hours
Barbiturates From minutes up to 48 hours
Benzodiazepines From minutes up to 48 hours
Cocaine From minutes up to 48 hours
Methadone From minutes up to 48 hours
Methamphetamine From minutes up to 48 hours
Opiates From minutes up to 48 hours
Oxycodone From minutes up to 48 hours
Phencyclidine From minutes up to 48 hours
THC (r-9-THC) From minutes up to 48 hours
Drugs of Abuse Testing — Oral Fluid
Routine laboratory oral fluid testing services
Levels updated periodically
Levels updated periodically
1. Detected by Enzyme-Linked Immunosorbent Assay (ELISA)
2. Cocaine Metabolite: Benzoylecgonine
3. Average detection times
These oral uid cutoffs are based upon preliminary guidelines established by the
Substance Abuse Mental Health Services Administration (SAMHSA) Drug Advisory
Board for drug testing of alternative matrices.
METHODOLOGY

laboratory services
10
FREQUENTLY ASKED QUESTIONS
How do I know oral uid testing is right for my agency?
Oral uid testing is easily used in a variety of testing arenas: Federal
& State Corrections, Clinics & Hospitals, Methadone Clinics, Federal,
State & County Probation, Counseling Centers, Physician Ofces,
Federal Halfway Houses, Drug Courts, Pre-employment, Behavioral
Health, Jails & Detention Centers, Rehabilitation & Treatment, Child &
Family Services, Mental Health and Schools & Universities.
Why should I implement oral uid testing at my facility?
Not only does oral uid testing save your agency time and money
in collection fees, it offers the convenience of testing for drugs of
abuse anywhere, at any time.
What are the testing and conrmation methodologies?
Specimens collected with the oral uid collection device are sent
to Redwood Toxicology Laboratory (RTL) for screening by Enzyme
Immunoassay (EIA) or Enzyme-Linked Immunosorbent Assay
(ELISA). Depending on account setup, positive screens, other than
methadone, are conrmed by gas chromatography/mass spectrom-
etry (GC/MS) or liquid chromatography-tandem mass spectrometry
(LC/MS/MS).
The analytical methods used by RTL for the detection of drugs of
abuse are scientically accepted and approved by the U.S. Depart-
ment of Health and Human Services.
What are the oral uid drug detection times?
The drug detection times in oral uid closely parallel those in blood.
In general, Amphetamines, Barbiturates, Benzodiazepines, Opiates,
Cocaine/Benzoylecgonine, Methadone, and PCP can be detected
for up to 48 hours
1
following use. Parent THC (marijuana) can be
detected in oral uid for up to 24 hours
1
.
I suspect that my donor just used a substance of abuse,
how long should I wait before collecting the specimen?
Depending on the drug and dosage, drugs may be detected in oral
uid in as little as a few minutes or up to approximately 2 hours
from the time of use.
Why are the drug levels in oral uids
lower than those in urine?
Oral uid drug levels largely correlate with the amount of drug in the
blood (dependent on the saliva/plasma ratio for each drug). Higher
drug and drug metabolite levels are found in urine because they are
concentrated by the kidneys during the excretion process.
How do I collect the oral uid specimen?
Refer to page 13-20 for specimen collection protocols. Instructions
for use are also included with your rst order of oral uid collec-
tion devices. Please read these instructions carefully. RTL offers
telephonic training. If you have questions, please call
800.255.2159, press option 1.
Can I ship my oral uid specimens
with my urine specimens?
RTL will accept both oral uid and urine specimens in the same
lab pack when sending ve or more specimens (e.g. three oral uid
specimens and two urine specimens). The specimens cannot, how-
ever, be mixed in the postage-paid mailer boxes due to U.S. Postal
Service regulations.
NOTE: It is important that the test request form is included with the
oral uid specimen. The specimen cannot be processed without the
information supplied on the test request form.
How long does RTL store the oral uid specimen?
RTL stores positive oral uid specimens for three (3) months in
frozen storage. Negative specimens are kept for 48 hours.
How will RTL report the oral uid results?
RTL offers reporting for the oral uid specimens via internet, U.S.
mail or facsimile. Please indicate your preferred method at the time
of account set-up.
What is the shelf life for the oral uid collection device?
The oral uid collection device has a minimum shelf life of 12 months.
Does OSHA classify oral uid as hazardous?
OSHA considers oral uid collections non-hazardous as long as the
specimen is not tinged with blood.
Who do I call to re-order the oral uid collection devices?
To request labels, collection bottles, and shipping materials, contact
our Supplies Department. Lab supply re-ordering is available to
existing clients with an account number.
Phone: 800.255.2159, press option 4.
E-mail: supplies@redwoodtoxicology.com
Web: https://www.redwoodtoxicology.com/resources/supply_form
Oral fluid drug testing continued
1. Average detection times

REFERENCE GUIDE // Laboratory services
11
OVERVIEW
Redwood Toxicology Laboratory (RTL) also offers a wide
range of specialized tests including: EtG/EtS Alcohol test-
ing, Synthetic Cannabinoid testing, Designer Stimulants
testing, Comprehensive drug testing, GHB testing, Fen-
tanyl testing and hCG (pregnancy) testing and more.
ETG/ETS ALCOHOL TESTING
EtG is a direct metabolite of alcohol (ethanol). Its presence in urine
may be used to detect recent ethanol ingestion, even after ethanol
is no longer measurable. The presence of EtG in urine is an indicator
that ethanol was ingested and can be detected in urine for up to 80
hours after ingestion.
In addition to EtG, recent scientic studies have identied ethyl
sulfate (EtS) as a second specic metabolite or biomarker of ethanol.
For this reason, RTL tests and reports EtS, in conjunction with EtG,
to conrm recent ethanol ingestion or exposure. The detection of EtG
and EtS offers greater sensitivity and accuracy for determination of
recent ethanol ingestion, than by detection of either biomarker alone.
SYNTHETIC CANNABINOID TESTING
Up to four times stronger than marijuana, synthetic cannabinoids
are deceptively marketed as herbal smoke or incense products.
With high positivity rates, synthetic cannabinoid tests have proven
to be an essential tool for a variety of treatment and criminal
justice situations.
In July 2012, the DEA banned synthetic cannabinoids based on
their structural classication, explicitly naming 15 chemicals, citing
numerous calls to poison control centers around the nation. In May
2013, the DEA placed a temporary ban on three additional synthetic
cannabinoid substances. However, newer generation compounds
continually emerge—making it more vital than ever to target syn-
thetic marijuana.
DESIGNER STIMULANT TESTING
Designer stimulants are sold online or available at smoke shops;
promoted as “bath salts,” “research chemicals,” or “plant food,”
product labeling attempts to circumvent regulation by suggesting
they are not for human consumption. Additionally, some forms of
designer stimulants may be sold as “legal” MDMA (Legal X), or sold
and veiled as MDMA tablets.
A new federal ban targets three designer stimulant compounds for
so-called bath salt drugs. This ban will help deter abuse and suf-
fering from these dangerous designer drugs. However, drug makers
will continue to develop new compounds to circumvent existing
drug laws. We are committed to providing timely and relevant tests
that enable you to make informed decisions. Now you’ll know.
COMPREHENSIVE DRUG TESTING
Conventional drug test panels will not detect the broad variety of
addictive prescription drugs. They pass undetected in standard test-
ing for such drugs as cocaine, marijuana, heroin and amphetamines.
RTL’s Comprehensive Drug Test solves this problem.
RTL’s Comprehensive Drug Test detects prescription drugs within a
variety of categories, including:
• Anticonvulsants
• Antidepressants/Analgesics
• Barbiturates
• Benzodiazepines
• Propoxyphene
• Sedatives/Hypnotic Agents
• Stimulants
RTL’s Comprehensive Drug Test also tests for alcohol, illicit drugs
and specimen validity.
• Alcohol
• Amphetamine
• Cocaine
• Marijuana
• Methadone
• Methamphetamine
• PCP
• Specimen validity (Creatinine)
GHB TESTING
There are increasing reports of Gamma-Hydroxybutyric acid (GHB)
being used recreationally as a euphoriant at “rave” type parties.
GHB is typically associated with sexual assault or as a “date rape”
drug due to its severe hypnotic and sedative effect at higher dos-
ages. Typical illicit use of GHB involves dissolving 2 - 3 grams of
powder in beverages.
STEROIDS/SPORTS DRUG TESTING
Due to popular demand, Redwood Toxicology Laboratory, Inc. (RTL)
developed a comprehensive and affordable steroid panel that is
comparable to WADA
1
testing. In addition, RTL also offers a diuretics
panel and stimulants panel.
Our tests are ideal for sports organizations, colleges and high
schools, certied athletic trainers, coaches, corrections and law
enforcement, and occupational health agencies
2
.
1. World Anti-Doping Agency
2. Compliance with RTL non-pretesting policy is required
Esoteric/Specialty Testing
Laboratory service


13
Specimen collection
Redwood Toxicology Laboratory provides suggested specimen collection guidelines only. It
is the responsibility of individual collection agencies to adopt their own policies and proce-
dures according to their needs in compliance with individual state and federal regulations.
Laboratory drug and alcohol test results are often used in legal proceedings. The manner
in which specimens are collected and handled is very important. Specimens must be
handled and controlled by collection site personnel throughout the collection process.
Directly observed urine collection is the best means to ensure specimen integrity. However,
outside of probation and parole and some drug rehab environments, the question of civil
rights arises. A uniform urine and oral uid collection process, regardless of the testing
environment, should be followed.
REFERENCE GUIDE // Specimen collection

specimen collection
14
PRINCIPLE
Requirements for specimen collection vary according to the purpose
for which the results will be used. However, to meet evidentiary
requirements the specimen collection site must be secure in order
to eliminate the possibility of specimen tampering or adulteration
and to ensure the security of the collected specimens.
COLLECTION AREA GUIDELINES
1. Storage area for collection supplies and related
materials is secure.
2. Collection site facility is secure, well lit, and free of
any areas where adulterants or substitute specimens
can be hidden.
3. A suitable clean surface for the collector to use as
a work area.
4. Eliminate or secure all sources of water in the area where
urination occurs. Bluing agent should be placed in the
toilet tanks and bowls to prevent sample dilution.
5. Eliminate or secure all soap or detergent dispensers or
any other potential adulterants.
6. A secured storage area should be available to ensure
specimen security prior to transport to the laboratory.
7. A general log book should be maintained to record
collected specimens.
Collection: Site Preparation
Suggested collection guidelines
Collection Area Example
REFERENCE GUIDE // Specimen collection
15
PRINCIPLE
The validity of urine drug screen results is dependent on specimen
integrity. While direct-observation collections provide specimens of
the greatest credibility, non-witnessed collections can be effective
if safeguards are in place to ensure the donor does not have access
to substances which may affect test results (water, chemicals,
substitute urine, etc.).
SUGGESTED PROCEDURE
PRIOR TO COLLECTION
Prior to any specimen collection procedure, secure the collection
facility (see Collection Site Preparation page 14) and if necessary,
perform a thorough search for hidden adulterants or substitute urine
specimens. Place bluing agent in the toilet bowl or tank, remove or
secure all chemicals (soaps, cleaning supplies, etc.) and secure or
eliminate all water sources.
1. Check the identity of donor
(e.g. social security number
or driver’s license number
and photo I.D.). If using a drug
screen test request form, note
the identity on the form.
2. Ask the donor to remove any
unnecessary outer clothing. All
personal belongings (the subject may retain a wallet) should be
placed in a secure location outside the stall or partitioned area.
3. Do not ask the donor to empty his/her pockets or to remove
articles of clothing such as shirts, pants, dresses, etc. If a
collector notices any unusual behavior that indicates a donor
may attempt to tamper with or adulterate a specimen (e.g.,
bulging pockets), the collector may request that the donor
empty his/her pockets and explain the need for such items
during collection.
4. Prior to collection, ask the
donor to wash his/her hands
to eliminate any possible
adulterating or contaminating
substances from under the
donor’s ngernails.
URINE COLLECTION PROCEDURE
1. Place the following information on the bottle label:
• Date of collection
• Donor’s name and/or identication numbers
• Collector’s initials
2. Provide the donor with a clean, unused urine specimen
collection container and instruct the donor to ll the
container at least half full (a minimum of 30 mL’s).
3. Unobserved Collection: Allow the donor to enter and maintain
privacy within the stall or partitioned area. The collector will
wait outside the collection area until the donor is nished
urinating. Complete the remainder of the test request form
while the donor is collecting the specimen. (See page 23-31
for detailed labeling instructions).
4. Observed Collection: Inform the donor that collection will
occur under direct observation. Accompany the donor into
the collection facility (the collector must be the same gender).
Instruct the donor to urinate into the sample container with the
witness observing urination. Complete the remainder of the
test request form after the donor has completed collecting the
specimen. (See page 23-31 for detailed labeling instructions).
5. Accept the specimen from the donor. The use of disposable
gloves is recommended when handling specimens, so prior
to accepting the specimen
from the donor, be sure to
wear gloves.
6. Upon receipt of the specimen
from the donor, immediately
apply the temperature strip (if
applicable) to the outside of the
bottle. If using a drug screen
test request form, record the urine
temperature on the form.
NOTE: Urine temperature should be measured within (4) four
minutes of collection and should read between 90-100ºF.
Collection: Urine/Oral Fluid Collection Protocol
Urine and oral fluid collection protocol
EXPIRES 00-00-00
CLASS: C
DRIVERS LICENSE
00000000
John Doe
123 Anywhere Ln.
Santa Rosa CA 95403
SEX:M HAIR:BLN EYES:BLU
HT:6-0 WT:190 DOB:01-01-65
Verify the donors identity
with a photo I.D.
Donors should wash hands before
donating specimens.
Apply temperature strip
to sample bottle.
specimen collection
16
ORAL FLUID COLLECTION PROCEDURE
1. Remove the kit contents from the packaging. Save the
outer packaging because the specimen must be placed
in the re-closable outer packaging for shipment to
the laboratory.
2. Peel open the collector pad pack-
age and remove the collection
device. Do not touch the pad.
3. Place the collector pad under the
donor’s tongue and instruct the
donor to close his/her mouth.
The donor must not chew or suck
on the pad. When the indicator
window turns blue, remove the
collection device from the donor’s
mouth. DO NOT remove the col-
lection device until the indicator
turns blue. If the indicator does
not turn blue within 15 minutes,
remove the collection device and
discard. Re-collection with a new
device may begin immediately
after saliva has accumulated in
the donor’s mouth.
4. Holding the transport tube in an upright
position, remove the cap, and insert
the collector device, pad rst, into the
tube. DO NOT set the transport tube on a
table. If any of the buffer uid is spilled,
a new transport tube must be used. The
amount of liquid in the transport tube is
critical to the testing process.
5. Push the cap rmly onto the transport tube
until you hear the SNAP. Gently shake the
tube to mix the saturated collector pad with
the buffer.
6. Complete the labeling procedure by following the instructions
on page 28 and 29.
Collection protocol continued
Peel open package and
remove collector.
Place collector pad under
the donors tongue.
Open transport tube and insert the
collector.
REFERENCE GUIDE // Specimen collection
17
PRINCIPLE
Methods to adulterate urine samples for substance abuse testing
generally fall into three categories; 1) urine substitution; 2) inges-
tion of uids or compounds for ushing out the system, diluting
the sample, or interfering with the
testing process; or 3) direct addition
of adulterants to the urine speci-
men itself. The substitution of one’s
own urine sample with one which
is clean is a common practice. The
best means to combat this practice
is to measure urine temperature,
as urine specimens even held close
to the body for extended periods of
time will not produce a physiologically
temperature-correct specimen. However, practices of reverse cath-
eterization with clean urine and placement of urine lled balloons in
the vaginal cavity can produce urines of correct temperature.
Drinking large volumes of liquid, especially cranberry juice or
vinegar is common practice. However, studies demonstrate these
practices have no effect on testing methodologies and may present
unexpected results.
Many of the drugs being tested are pH dependent. When large
volumes of cranberry juice or vinegar are consumed, the urine pH
is lowered, and the excretion rate of these drugs may increase. If
timed correctly, large amounts of a drug may appear sooner in
the sample.
Be aware, drinking large volumes of vinegar can be toxic.
One potentially effective method
which may negatively impact the
testing process is to consume large
volumes of water, as short term
water loading can increase urine
volume up to eight fold. Therefore,
if the individual‘s drug concentra-
tion is near the cutoff of an assay,
the urine may be diluted enough
so that the sample will test below the
cutoff level. Other attempted methods of
adulteration include ingesting large amounts of vitamin C, vitamin B,
niacin, Golden Seal, etc. All of these practices are ineffective.
Adulteration of a urine sample with various chemicals is shown (in
the literature) to inactivate some of the laboratory testing meth-
odologies, most notably, the enzyme immunoassay‘s. Addition of
compounds such as sodium chloride, sodium bicarbonate, hydrogen
peroxide, bleach, alcohols, blood, various soaps, etc. are shown to
produce both false negatives and false positives.
The current list of urine adulterants is ever changing as the Internet
provides an informational source, as well as a retail outlet for com-
mercial products capable of affecting the outcome of some urine drug
testing methodologies. Currently, nitrites (Klear and Whizzies) and
chromates (Urine Luck) are two adulterating agents commonly found
in the industry. RTL is capable of providing testing for some of
these agents.
Collection: Specimen Validity
Specimen tampering/adulteration
Human urine specimens should
range between 90º and 100º F.
Consuming too much uid prior
to collection can result in an
invalid specimen.

specimen collection
18
ADULTERATION & DILUTION DETECTION
Means to detect adulteration by the collector and/or the laboratory
include the following:
1. Specimen Temperature: If specimen collection is not
witnessed, the most effective means to detect specimen dilu-
tion, adulteration, or substitution is to measure the sample’s
temperature. The collector should measure the temperature
utilizing the temperature strip afxed to the specimen con-
tainer within four minutes of collection; it should read between
90 and 100 degrees Fahrenheit. The urine temperature should
be noted on Urine Test Request form.
2. Urine Appearance and Odor: Adulterants such as isopro-
pyl alcohol, soaps, bleach and perfumes are readily identied
by their odor. Soaps are also identied by excessive bubbling.
Use of solid adulterants is detected by the presence of residues
in the container.
3. Creatinine: In general, creatinine is a metabolic byproduct
of muscle metabolism which normally appears in urine in rela-
tively constant quantities over each 24 hour period. Therefore,
urine creatinine can be used both as a marker to specically
identify a specimen as urine, and as an indicator of urine water
content (dilution). “Normal” random urine specimens will
generally have urine creatinine levels of greater than 20 mg/dL,
while specimens with creatinine levels between 10 and 20 mg/
dL may be due to increased liquid consumption, dietary habits,
or liquid ingestion preferences. Urine specimens with creatinine
levels between 2 and 10 mg/dL are usually a result of ingestion
of large volumes of water (or other liquid), termed short term
water loading. This is a very common practice when attempting
to dilute a urine so that any drugs in the urine will be diluted
below analytical testing cutoff levels. Urine creatinine levels
below 2.0 mg/dL are usually a result of “dipping”, the direct
addition of a liquid to the urine specimen. Creatinine levels of
0.0 mg/dL indicate the specimen is not consistent with
human urine.
Urine specimens become dilute as a result of the short term
consumption of large amounts of a liquid due to an unknown
stimulus such as a response to heat or exercise, herbal ushes,
prescription diuretics, intentional dilution, or pathological
situations such as diabetes insipidus. The dilution effect from
consuming increased volumes of liquid can last from 2 – 5
hours. Therefore, the increased consumption of liquid would
have to take place between 2 – 5 hours prior to the collection
of the urine specimens.
An important factor to consider when interpreting dilute urine
samples is that drug use can never be assumed unless speci-
cally detected, and conrmed in a urine sample. Certainly, a
dilute sample can produce false negative results, as drugs in
the urine at concentrations near the testing cutoff may be
diluted below the testing cutoff level, however, due to the
reasons stated above it can be difcult to establish the reason
or intent for the sample(s) being dilute. For these reasons, urine
creatinine is reported in conjunction with testing for drugs of
abuse, as an indicator of specimen validity only, as urine
specimens with a creatinine level below 20 mg/dL may have
an increased likelihood of producing a false negative drug
testing result.
For more information about creatinine, refer to page 46.
4. Specic Gravity: Normally, a random urine specimen will
have a specic gravity of greater than 1.003
1
. An extremely
low specic gravity (<1.003) indicates a dilute specimen,
while abnormally high specic gravity (>1.045) may indicate
the presence of dissolved solids such as sodium chloride and
sodium bicarbonate.
5. pH: Normal random urine pH is 4.8-7.8*. Low pH’s indicate pos-
sible ingestion of acidic substances such as cranberry juice or
vinegar; starvation; diarrhea; or direct adulteration of the speci-
men itself with acidic compounds. Elevated pH’s may indicate
the presence of basic compounds such as sodium bicarbonate,
bleach, or Drano; vegetarian diet; or prolonged vomiting. pH
levels of <3 or ≥11 are consistent with adulteration.
6. Visible Blood: Indicates the presence of blood in the urine
specimen. The presence of blood in the urine sample may
adversely impact the testing process and, in addition,
constitutes a biohazard for laboratory employees. Collection
of clean catch urine specimens during menstruation should
be attempted.
Urine adulteration is a double edged sword as both false negative
and false positive results can occur. However, most adulteration
attempts can be detected by either trained collection site person-
nel or by collection procedures as outlined above. Coordination and
cooperation between the collection site and the testing laboratory
provides effective and reliable drugs of abuse testing.
1. Normal ranges are indicated for freshly voided urines only.
Specimen tampering/adulteration continued

REFERENCE GUIDE // Specimen collection
19
PRINCIPLE
While most donors will cooperate fully if treated with dignity and
courtesy, there may be instances when unusual events may occur.
For this reason, it is imperative for the collector to have a thorough
understanding of the collection process and to have the ability to
explain the process clearly to the donor so they will fully understand
the directions. During collection of the specimen and completing
the forms, it is vital that the collector devote his/her full attention
to the procedure without interruptions. Any unusual appearance or
behavior is to be noted on the urine chain of custody form.
PROCEDURE
While it is not possible to anticipate every type of unusual event,
some of the more frequent are:
1. Specimen Temperature Outside Limits: If the specimen tem-
perature is outside normal parameters, i.e., less than 90ºF or
greater than 100ºF then:
A. Inform the donor that the temperature of the specimen
is outside normal limits (too low or too high) and that the
specimen needs to be recollected. If the specimen cannot
be recollected or the donor refuses, inform the donor that
specimen temperature will be noted on the nal report.
NOTE: This is important because if the donor has drug
in his/her system at the time of collection, collection at a
later time may allow the drug to clear. Therefore, if at all
possible, make every effort to resolve the situation at the
time of the incident.
2. Specimen Contains Visible Blood: Urine specimens may not
contain visible blood for drug testing because:
• Blood may interfere with the testing process (which could
result in false negative tests).
• Urines containing visible blood are considered biohazard-
ous and require special packing procedures in order to be
shipped (Federal Bloodborne Pathogens standard).
Instructions to client to obtain a clean catch
urine specimen:
A. Wipe area until free of blood.
B. Start urinating into the toilet. After a few seconds, place
the urine container under the stream of urine until the cup
is at least half-full.
3. Uncooperative or Belligerent Donor: Presumably, the donor
has agreed to the drug test prior to appearing at the collection
site. However, attitudes may change just before, or during the
collection process. Remain courteous and do not argue with
a donor. It remains the right of the donor to refuse collection
at any time, of course at his/her own risk. Remind the donor
that you will call the requesting agency with a recount of the
pertinent facts.
If physical violence seems imminent, call for assistance and
ask the donor to leave the premises. Use the same procedure
you would use for any other circumstance in which you fear
bodily harm or property damage, including calling 911.
4. Suspicion Donor is Adulterating Sample (Adding water
or other substance): Remain courteous and do not
argue with the donor. The following statement may
be appropriate:
“We are instructed to tell everyone that the lab tests
for water and other materials that may have been added.
It will show up on the test report.”
Collection: Problematic Situations
Donor situations

specimen collection
20
Grounds for these type of suspicions may include out of range
specimen temperature, abnormal urine smell or
appearance, or unusual sediments.
5. Donor Cannot Urinate or Produces Insufcient Volume: Upon
receipt of the specimen, the collector must rst determine if
there is sufcient urine for testing. Minimum sample volume
is twenty (20) mL and is sufcient for retest and conrmatory
procedures if required. If there is not sufcient urine volume,
then follow the procedures below:
A. Ask a supervisor to determine if there is adequate urine
volume to perform the requested testing.
B. If there is not a sufcient urine volume, the collector shall
take possession of the partial specimen and instruct the
donor to drink uids (no more than 8 oz.) and try again in
a reasonable amount of time. If possible, the donor should
remain on the premises and preferably within visual
contact of the collection site person until a complete
specimen is provided.
C. In the event a donor cannot provide a specimen of ade-
quate volume, the requesting agency should be notied for
further instruction. In some cases, it may be acceptable to
reschedule the collection. However, it may be necessary to
determine whether a valid medical reason exists for the
donor’s scarce urine output or if the donor is refusing to
provide a specimen.
6. Donor Accuses Collector of Carelessness, Personal Miscon-
duct, or Deliberate Mishandling of Specimen: This is unlikely;
however it does occur.
Remain calm and professional. Listen carefully. If there is
another person at the site, ask that individual to join you and
the participant. If you are alone, call your supervisor while in
the presence of the participant. It is important to document
what took place and what was said. The matter should be
treated with the utmost seriousness. It could result in the loss
of a client, a civil lawsuit, or even a criminal suit. State fully
to the participant and, if possible, to a witness what you did
or did not do. Make every reasonable effort to persuade the
participant of your good intentions and lack of negligence;
however, do not attempt to deny actual error on your part. If it
is obvious that a participant is attacking you to perhaps cover
the presence of drug use, treat it as a legal matter with docu-
mentation and immediate notication to your supervisor.
Donor situations continued

REFERENCE GUIDE // Specimen collection
21


23
Specimen labeling & shipping
Redwood Toxicology Laboratory (RTL) provides specimen collection guides on how to prop-
erly label and package specimens being sent to RTL for screening & conrmation testing.
Each test requisition form requires unique labeling and it is important that these guidelines
are followed to ensure proper specimen processing at RTL. Mislabeled specimens can delay
processing and reporting timelines.
REFERENCE GUIDE // Specimen labeling & shipping

specimen collection
24
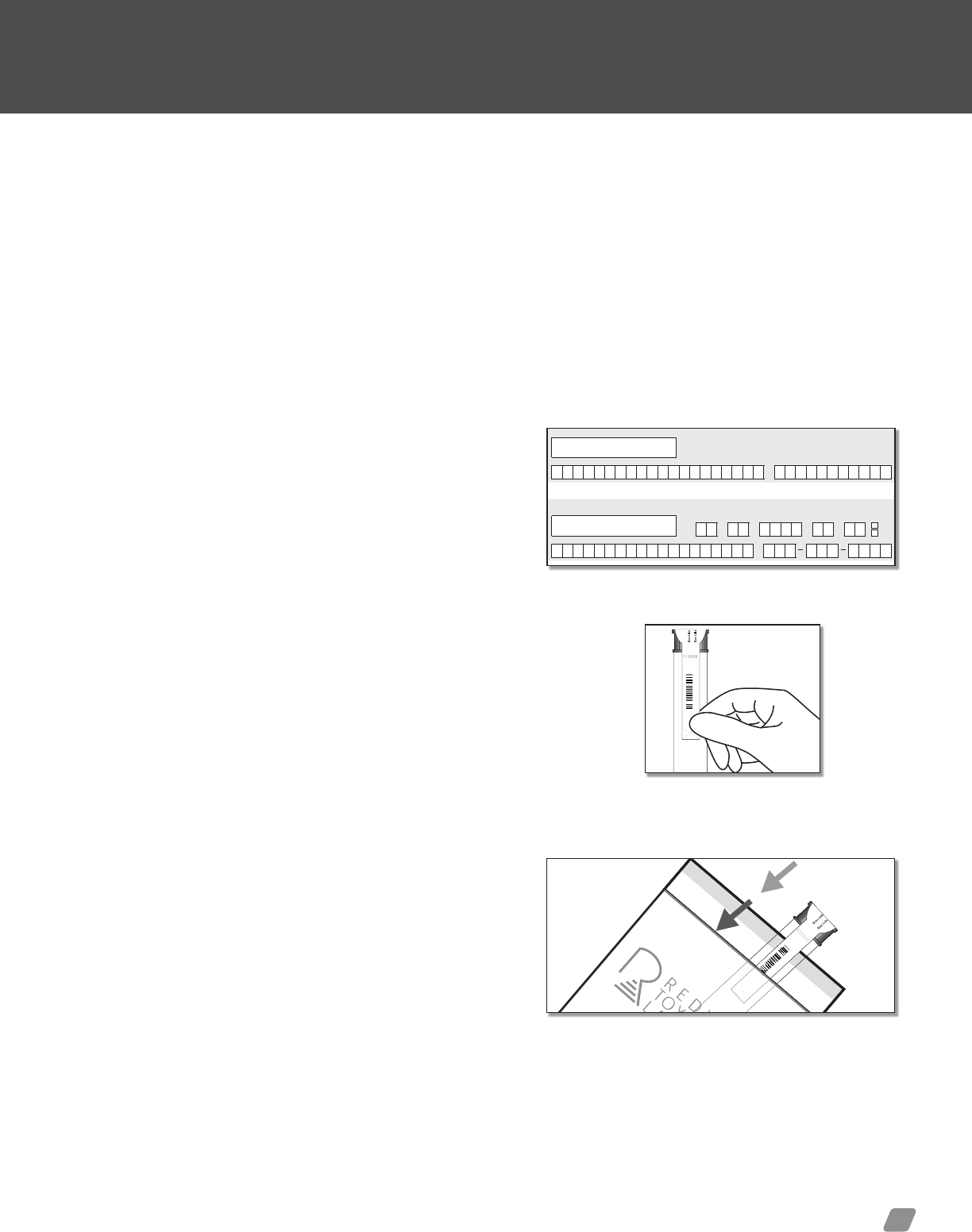
Option 1: ToxAccess
™
Form
ToxAccess
™
test requisition form overview
TOXACCESS
™
TEST REQUISITION FORMS
1) Agency Information—Contains the agency’s account
information including: account number, name, address,
phone and fax numbers.
2) Donor Information & Test(s) Requested—Donor informa-
tion will be located in this section. You will also see the tests
requested to the right of the donor’s information.
3) Specimen Verication—Signature area for Donor and Collec-
tor, verifying specimen collected and labeled correctly.
4) Security Seal—Peel off label secures specimen container.
5) Specimen Label—This label will contain all required informa-
tion for the laboratory to process your specimen. Ensure the
label is placed horizontally across the specimen bottle.
6) Receiving (lab only)—To be lled out by RTL personnel only.
7) Donor Receipt—The tear off section of the chain of custody
form is to be provided to the donor for their records.
For training or questions, please contact:
Toxicology Support Services
Phone: 800.255.2159, press option 5.
Fax: 707.577.0365
Email: clientservices@redwoodtoxicology.com
Web: https://www.redwoodtoxicology.com/services/online_reporting
The ToxAccess
™
Collection Management system accelerates donor
scheduling, data entry, and the test ordering process.
The online data you input is automatically transferred into RTL’s
laboratory information system, eliminating both the errors caused
by hand-written labels, and laboratory data entry errors.
Following the data input process; administrators print ToxAccess chain
of custody forms using special paper supplied by RTL. Each printed
form features a unique test request label and specimen security seal.
The printed form will be signed with a water-resistant marker, such as
a blue or black ball-point pen (red color is not recommended since it
tends to rub off).
Print your own test request forms
with paper provided by RTL.
Only available with ToxAccess.
ToxAccess
™
Test Request Form
Laboratory Drug Test
Test Requisition Form
Tear off and provide to donor
11 000 3313 REV3
an Alere company.
AGENCY INFO:
Web Demo Agency
3650 Westwind Blvd
Santa Rosa, CA 95403
Phone: (800)255-2159
Fax:
COLLECTION INFORMATION:
Donor Name: John J. Doe
Requisition #: 000000000
Test Reason: RANDOM
Collection Date: 6/29/2011
Collection Time: 10:00 AM
LABORATORY STAFF ONLY
RECEIVED
AT LAB:
Seal Intact?
X
Received By (initial) Date
Labels Match?
Agency Name: Web Demo Agency
Address: 3650 Westwind Blvd.
City, State and Zip: Santa Rosa, CA 95403
Agency Phone: (800)255-2159
Agency Fax:
Agency Number: 600000
000000000
DONOR INFORMATION
Donor Name: John J. Doe
Donor Unique ID: 54321
Specimen Type: URINE
Specimen Temperature: 97F
Test Reason: RANDOM - 3
Tests Requested:
647 - Ethylglucuronide (EtG)
P08 - Screen 7 with Creatinine
I certify that this specimen was collected from the above person following established protocols, and the specimen has been properly sealed and labeled.
COLLECTOR VERIFICATION
X
Collector’s Signature Collector’s Name Collection TimeDate
Jim J. Smith 6/29/2011 10:00 AM
Donor’s Name Date
I certify that I provided my specimen to the collector and that I have not adulterated it in any manner. The specimen was sealed in my presence with a tamper-evident
seal. The information provided on this form and on the label affixed to the specimen container is correct. I authorize Redwood Toxicology Laboratory to perform the tests
listed and to release the results of this test to WEB DEMO AGENCY
DONOR CERTIFICATION
X
Donor’s Signature
John J. Doe 6/29/2011
Yes No Yes No
1. Tighten cap.
2. Place security seal across
the lid as shown.
3. Place patient I.D. label around
bottle as shown.
Label usage example
Security
Seal
Security Seal
0000 000 0
Req #: 000000000
Agency: 600000—Web Demo Agency
ID: 54321 John J. Doe
Collector: Jim J. Smith
Collected: 6/29/2010 10:00 AM
Tests: 647, P08
Donor’s Initials
Date:
Redwood Toxicology Laboratory // 3650 Westwind Blvd. Santa Rosa, CA 95403 | (800) 255-2159
6/29/2011
1
4
1
2
6
7
3
4
5
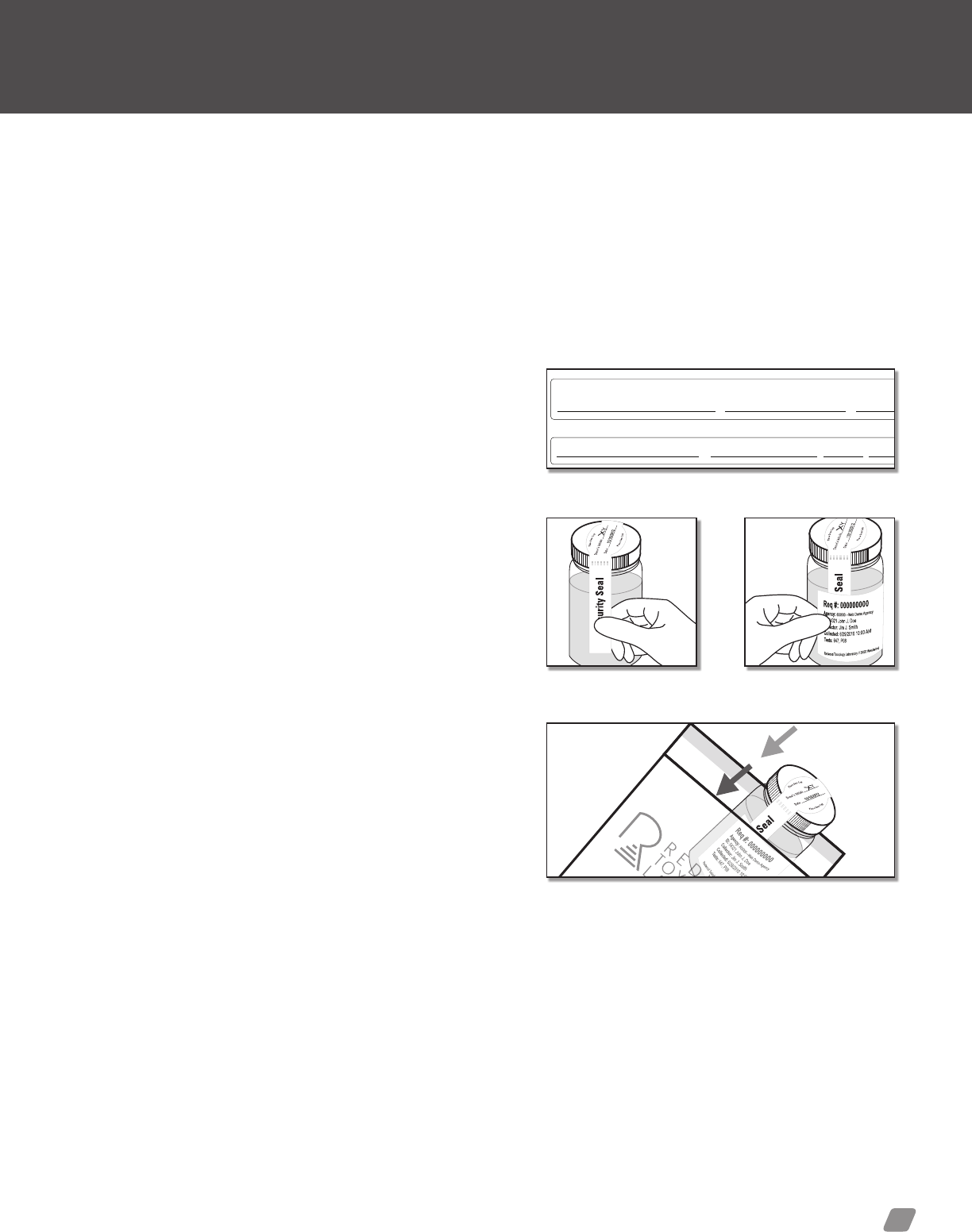
REFERENCE GUIDE // Specimen collection
25
ToxAccess
™
: Labeling Protocol
How to label specimens using the ToxAccess
™
forms
LABELING OVERVIEW
For customers taking advantage of the web-based ToxAccess
™
Collection Management solution. Please follow these labeling
instructions. Correctly labeled specimens will allow the laboratory
to process your specimens accurately and quickly.
LABELING PROCEDURE
1) Seal container—After the collection process is complete,
ensure the specimen container is tightly sealed.
2) Sign and date the form—The donor will certify that the speci-
men was collected following the appropriate procedures by
signing under the “Donor’s Signature” area. The collector will
also sign the form verifying that the specimen was collected
appropriately in the “Collector Verication” area.
3) Place security seal on the specimen bottle—Once the speci-
men has been veried by the donor and collector, the collector
will remove the security seal from the form and place it across
the top of the bottle. The donor will then initial security seal.
4) Place specimen label on the specimen bottle—Place
the specimen label around the body of the specimen bottle.
The label should lay over the ends of the security seal as
represented in the images to the right. Do not hand write any
tests on the label. Tests may only be ordered through the
ToxAccess program.
5) Package for shipping—After labeling and sealing the speci-
men tightly, place it into a RTL branded plastic baggie with
absorbent material. Ensure baggie is sealed. Store in a
secure area until the specimen is ready to be shipped to
the laboratory.
Laboratory Drug Test
Test Requisition Form
Tear off and provide to donor
11 000 3313 REV3
an Alere company.
AGENCY INFO:
Web Demo Agency
3650 Westwind Blvd
Santa Rosa, CA 95403
Phone: (800)255-2159
Fax:
COLLECTION INFORMATION:
Donor Name: John J. Doe
Requisition #: 000000000
Test Reason: RANDOM
Collection Date: 6/29/2011
Collection Time: 10:00 AM
LABORATORY STAFF ONLY
RECEIVED
AT LAB:
Seal Intact?
X
Received By (initial) Date
Labels Match?
Agency Name: Web Demo Agency
Address: 3650 Westwind Blvd.
City, State and Zip: Santa Rosa, CA 95403
Agency Phone: (800)255-2159
Agency Fax:
Agency Number: 600000
000000000
DONOR INFORMATION
Donor Name: John J. Doe
Donor Unique ID: 54321
Specimen Type: URINE
Specimen Temperature: 97F
Test Reason: RANDOM - 3
Tests Requested:
647 - Ethylglucuronide (EtG)
P08 - Screen 7 with Creatinine
I certify that this specimen was collected from the above person following established protocols, and the specimen has been properly sealed and labeled.
COLLECTOR VERIFICATION
X
Collector’s Signature Collector’s Name
Collection Time
Date
Jim J. Smith 6/29/2011
10:00 AM
Donor’s Name Date
I certify that I provided my specimen to the collector and that I have not adulterated it in any manner. The specimen was sealed in my presence with a tamper-evident
seal. The information provided on this form and on the label affixed to the specimen container is correct. I authorize Redwood
Toxicology Laboratory to perform the tests
listed and to release the results of this test to WEB DEMO AGENCY
DONOR CERTIFICATION
X
Donor’s Signature
John J. Doe 6/29/2011
Yes No Yes No
1. Tighten cap.
2. Place security seal across
the lid as shown.
3. Place patient I.D. label around
bottle as shown.
Label usage example
Security
Seal
Security Seal
000 000 00
Req #: 000000000
Agency: 600000—Web Demo Agency
ID: 54321 John J. Doe
Collector: Jim J. Smith
Collected: 6/29/2010 10:00 AM
Tests: 647, P08
Donor’s Initials
Date:
Redwood Toxicology Laboratory // 3650 Westwind Blvd. Santa Rosa, CA 95403 | (800) 255-2159
6/29/2011
Donor signature and collector signature lines.
Security seal placed
across the specimen cap.
Place specimen label
over the security seal.
Place urine specimen into the plastic baggie.
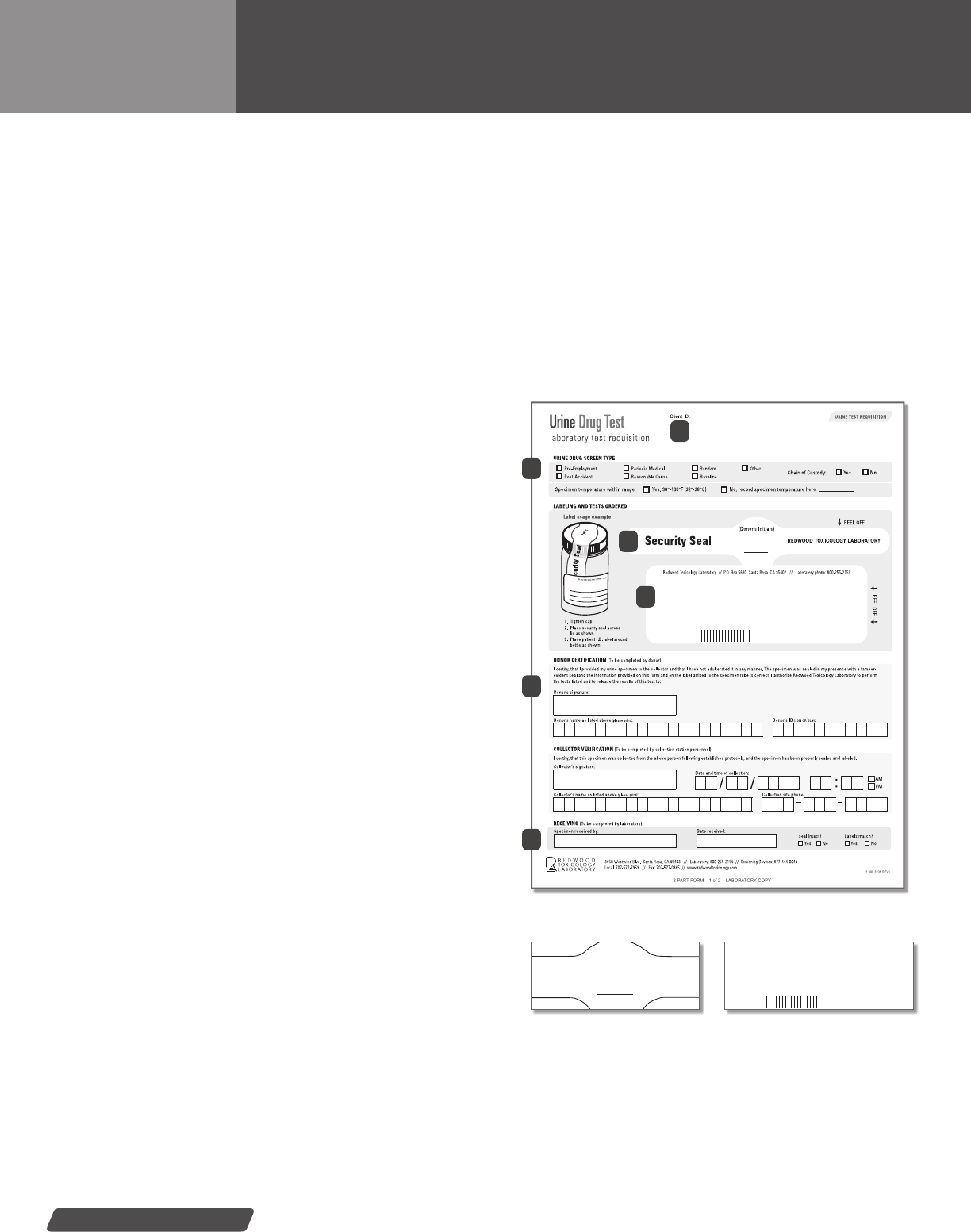
specimen collection
26
Option 2: Urine Test Request Form
Multi-part urine test requisition form overview
[X] P08/1108 Screen 8
[ ] 098 Oxycontin ($5.00)
[ ] Other: Collector: _________
–––––––––––––––––––––––––––––––––––––––––––––––––––––––––
GC/MS Confirm ______________________
(Specify Drug(s))
Patient ID: _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ Collection Date _ ________
Req#
777777
P.O. Box 5680 Santa Rosa, CA 95403 // Laboratory phone: 800-255-2159
Marijuana
John Smith
10/20/07
A completed chain of custody labelAn initialed security seal
REDWOOD TOXICOLOGY LABORATORY
Security Seal
(Donor’s Initials)
JS
URINE TEST REQUISITIONS
FORMS (RF2/RF3)
1) Agency Information—Contains the agency’s account
information including: account number, name, address,
phone and fax numbers.
2) Urine Drug Screen Type—Indicates multiple boxes with
corresponding reasons for testing the donor
*
. Specimen
temperature is also noted in this area.
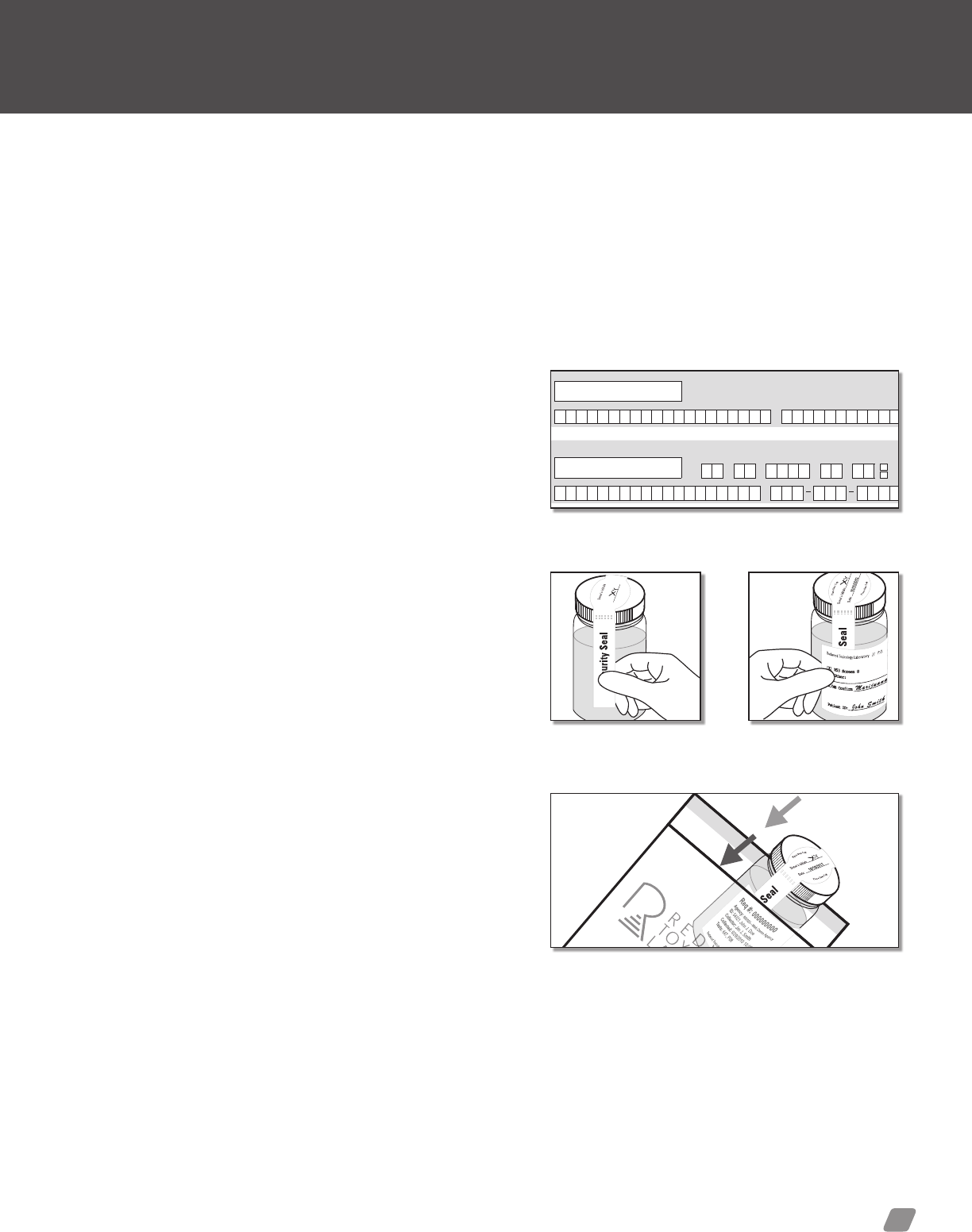
3) Security Seal—Peel off label secures specimen container.
4) Specimen Label—The collector will select the a laboratory
test from the available options or specify the desired test in the
“other” eld. Collector will then enter the donor’s ID, collector
initials and collection date onto the label. Ensure the label is
placed horizontally across the specimen bottle.
5) Donor Information & Collector Verication—Donor informa-
tion will be located in this section. Signature area for Donor and
Collector, verifying specimen collected and labeled correctly.
6) Receiving (lab only)—This section is to be lled out by
Redwood Toxicology Laboratory personnel only.
* Donor identication (Name, ID Number, Etc.) will appear on the nal report.
Donor ID must be written on the Patient ID line of the label. It is recommended
that the donor’s Social Security Number not be writen on the Patient ID line.
9999
Demo Agency
3650 Westwind Blvd.
Santa Rosa, CA 95403
Phone: 707-577-7959
[ ] P08/1108 Screen 8
[ ] 098 Oxycontin ($5.00)
[ ] Other: Collector: _________
–––––––––––––––––––––––––––––––––––––––––––––––––––––––––
GC/MS Confirm ______________________
(Specify Drug(s))
Patient ID: _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ Collection Date _ ________
Req#
777777
Urine Test Request Form
Urine Test Requisition forms will be provided when ordering urine tests
through the lab. The Urine Test Requisition Forms (RF2 and RF3) are
chain of custody labels with either 2 or 3 part carbon copies.
The forms should be completed with a water-resistant marker, such
as a blue or black ball point pen (red color is not recommended since it
tends to rub off).
1
2
5
6
3
4
For training or questions, please contact:
Toxicology Support Services
Phone: 800.255.2159, press option 5.
Fax: 707.577.0365
Email: clientservices@redwoodtoxicology.com
Web: https://www.redwoodtoxicology.com/services/online_reporting
REFERENCE GUIDE // Specimen collection
27
Urine Test Request Form (RF2/RF3): Labeling Guide
How to label specimens using the RF2/RF3 multi-part forms
LABELING OVERVIEW
The following procedure gives you an easy to follow guide to ensure
your specimens are labeled correctly using the RF2/RF3 test request
forms. Correctly labeled specimens will allow the laboratory to pro-
cess your specimens accurately and quickly.
LABELING PROCEDURE
1) Seal container—After the collection process is complete,
ensure the specimen container is tightly sealed.
2) Sign and date the form—The donor will certify that the speci-
men was collected following the appropriate procedures by
signing under the “Donor’s Signature” area. The collector will
also sign the form verifying that the specimen was collected
appropriately in the “Collector Verication” area.
3) Place security seal on the specimen bottle—Once the speci-
men has been veried by the donor and collector, the collector
will remove the security seal from the form and place it across
the top of the bottle. The donor will then initial security seal.
4) Indicate the test(s) to be performed—Indicate the following
information in the appropriate area of the specimen label:
• Please indicate which test(s) or panel is to be ordered by
placing a check mark in the appropriate box or by writing the
test on the “other” line. Tests to be run by GC/MS or LC/MS/
MS must be written on the GC/MS request line.
• Donor identication, collection date, and collector.
5) Place specimen label on the specimen bottle—Place the
specimen label around the body of the specimen bottle. The
label should lay over the ends of the security seal as represent-
able in the images to the right.
6) Package for shipping—After labeling and sealing the
specimen tightly, place it into a RTL branded plastic baggie
with absorbent material. Ensure baggie is sealed. Store in a
secure area until the specimen is ready to be shipped to
the laboratory. The test request form (chain of custody form)
must be placed in the same shipping bag as the specimen.
If the form is sent in a separate bag, it will not be matched to
the specimen.
Chain of Custody:
Yes No
URINE DRUG SCREEN TYPE
Pre-Employment
Post-Accident
Periodic Medical
Reasonable Cause
Random
Baseline
Other
Specimen temperature within range:
° ° ° °Yes, 90 -100 F (32 -38 C) No, record specimen temperature here
DONOR CERTIFICATION (To be completed by donor)
I certify, that I provided my urine specimen to the collector and that I have not adulterated it in any manner. The specimen was sealed in my presence with a tamper-
evident seal and the information provided on this form and on the label affixed to the specimen tube is correct. I authorize Redwood Toxicology Laboratory to perform
the tests listed and to release the results of this test to:
Donor’s name as listed above (please print): Donor’s ID (SSN OR DL #):
Donor’s signature:
Collector’s name as listed above (please print): Collection site phone:
COLLECTOR VERIFICATION (To be completed by collection station personnel)
I certify, that this specimen was collected from the above person following established protocols, and the specimen has been properly sealed and labeled.
Collector’s signature:
Date and time of collection:
/ /
:
AM
PM
RECEIVING (To be completed by laboratory):
Seal intact?
Yes No
Labels match?
Yes No
Specimen received by: Date received:
LABELING AND TESTS ORDERED
Label usage example
1. Tighten cap.
2. Place security seal across
lid as shown.
3. Place patient I.D. label around
bottle as shown.
PEEL OFF
PEEL OFF
REDWOOD TOXICOLOGY LABORATORY
Security Seal
(Donor’s Initials)
Redwood Toxicology Laboratory // P.O. Box 5680 Santa Rosa, CA 95402 // Laboratory phone: 800-255-2159
Security
Seal
P
.
O
.
B
o
x
5
6
8
0
S
a
n
t
a
R
o
s
a
,
C
A
9
5
4
0
3
/
/
L
a
b
3650 Westwind Blvd. Santa Rosa, CA 95403 // Laboratory: 800-255-2159 // Screening Devices: 877-444-0049
Local: 707-577-7959 // Fax: 707-577-0365 // www.redwoodtoxicology.com
11 000 3206 REV1
URINE TEST REQUISITION
Urine Drug Test
laboratory test requisition
Donor signature and Collector Signature lines.
Security seal placed
across the specimen cap.
Place specimen label
over the security seal.
Place urine specimen into the plastic baggie.
specimen collection
28
Option 3: Oral Fluid Specimens
Multi-part oral fluid test requisition form overview
Oral Fluid Test Request Form
Chain of Custody:
Yes No
ORAL FLUID DRUG SCREEN TYPE
Pre-Employment
Post-Accident
Periodic Medical
Reasonable Cause
Random
Baseline
Other
Collector’s name as listed above (please print): Collection site phone:
COLLECTOR VERIFICATION
(To be completed by collection station personnel)
I certify that this specimen was collected from the above person following collection site protocols, and the specimen has been properly sealed and labeled.
Collector’s signature:
Date and time of collection:
/ /
:
AM
PM
RECEIVING
(To be completed by laboratory):
Seal intact?
Yes No
Labels match?
Yes No
Specimen received by: Date received:
3650 Westwind Blvd. Santa Rosa, CA 95403 // Laboratory: 800-255-2159 // Screening Devices: 877-444-0049
Local: 707-577-7959 // Fax: 707-577-0365 // www.redwoodtoxicology.com
11 000 3164 REV4
2-PART FORM 1 of 2 LABORATORY COPY
DONOR CERTIFICATION
(To be completed by donor)
I certify that I provided my oral fluid specimen to the collector and that I have not adulterated it in any manner. The specimen was sealed in my presence with a tamper-
evident seal and the information provided on this form and on the label affixed to the specimen tube is correct. I authorize Redwood Toxicology Laboratory to perform
the tests listed and to release the results of this test to:
Donor’s name as listed above (please print): Donor’s ID (SSN OR DL #):
Donor’s signature:
99999 - ANY AGENCY
ORAL FLUID SPECIMEN LABELING
10000 00001
ORAL FLUID TEST REQUISITION
Oral Fluid Drug Test
laboratory test requisition
99999
ANY AGENCY
1111 Anywhere Ln.
Suite 100
Anywhere, CA 95403
Test Panel: 9003 (AMP, COC, m-AMP, OPI, PCP, THC)
GC/MS Confirm:_____________________________________
Donor Initials
Date Collected
Security Seal
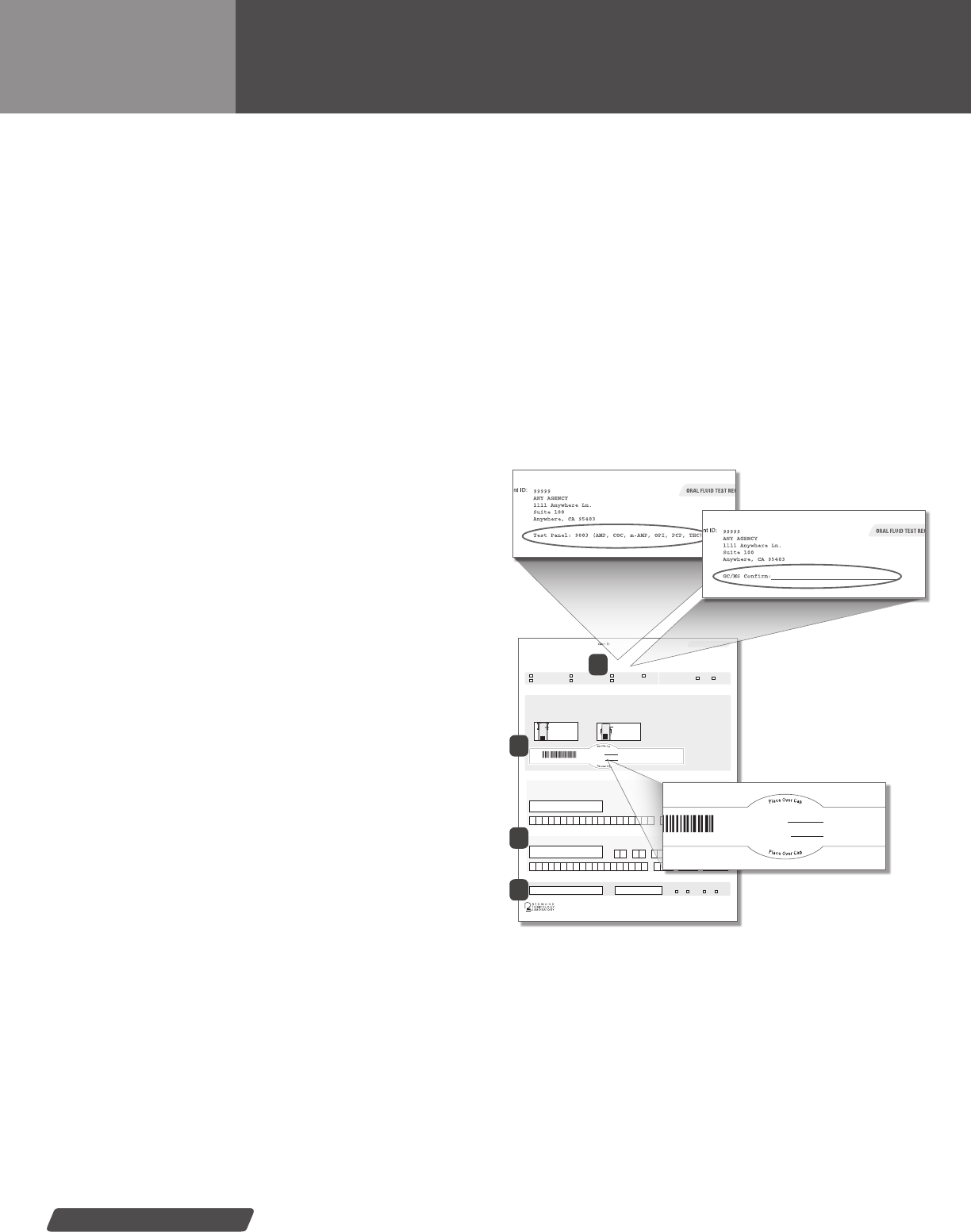
REDWOOD TOXICOLOGY LABORATORY
NOTE: Place the center of this
label over the cap of the screening
device or collection device.
10000 00001
LAB BASED COLLECTOR INSTRUCTIONS:
Ensure red cap is tightly fastened. Place the center
of the security seal over the top of the red cap. Be
sure that the barcode runs lengthwise/vertically on
the collection tube.
RTL-Oral Labeling Example
0000000
Security seal
with barcode
APPLY AS SHOWN
Donor initials
Date collected
ON-SITE SCREENING DEVICE EXAMPLE:
Ensure the screw cap is tightly fastened. Write the drug(s) to be
confirmed on the GC/MS confirm line above. Place the center
of the security seal over the top of the cap. Be sure that the
barcode runs lengthwise/vertically on the collection tube.
On-site Screening Device
Security Seal
0000000
Security seal
with barcode
APPLY AS SHOWN
Donor Initials
Date Collected
Security Seal
REDWOOD TOXICOLOGY LABORATORY
10000
00001
JD
10/20/09
Security Seal & Barcode—
A completed oral uid label
1A: Lab Tests—Agency specic drug test panel listed
1B: On-site Devices—List drug(s)
to be conrmed for your
on-site screening device
ORAL FLUID SPECIMEN
TEST REQUISITIONS
1) Lab Test Request Information—Lists agency specic test
panel information. Test(s) to be performed will be printed on the
top of the form. Indicate the panel and/or additional test to be
run by placing a check mark next to the panel or test descrip-
tion. (Diagram 1A)
On-site Device Test Request Information—Use the “GC/MS
Conrm” line to list the presumptive positive drug(s) you want
to conrm through the lab. Please note: panel cannot be run on
on-site devices. (Diagram 1B)
Check one of the boxes for why the donor is being tested in
the green box below the test request information.
2) Security Seal with barcode—Peel off label secures
specimen container.
3) Donor Information & Collector Verication—Donor informa-
tion will be located in this section. Signature area for Donor and
Collector, verifying specimen collected and labeled correctly.
4) Receiving (lab only)—This section is to be lled out by
Redwood Toxicology Laboratory personnel only.
Oral Fluid Test Requisition forms will be provided when ordering oral
uid tests through the lab. These forms are different from the Urine
Test Requisition forms and are therefore not interchangeable. Please
send the appropriate form with the specimen. If the laboratory copy
of this form is not sent with the specimen, the lab will be unable to
process the specimen. This form is a two-part carbon copy.
Ensure that the security seal, dates and appropriate signatures are
completed by the donor and the collector.
The forms should be completed with a water-resistant marker, such
as a blue or black ball point pen (red color is not recommended since it
tends to rub off).
1
2
3
4
For training or questions, please contact:
Toxicology Support Services
Phone: 800.255.2159, press option 5.
Fax: 707.577.0365
Email: clientservices@redwoodtoxicology.com
Web: https://www.redwoodtoxicology.com/services/online_reporting
REFERENCE GUIDE // Specimen collection
29
Chain of Custody:
Yes No
ORAL FLUID DRUG SCREEN TYPE
Pre-Employment
Post-Accident
Periodic Medical
Reasonable Cause
Random
Baseline
Other
Collector’s name as listed above (please print): Collection site phone:
COLLECTOR VERIFICATION (To be completed by collection station personnel)
I certify that this specimen was collected from the above person following collection site protocols, and the specimen has been properly sealed and labeled.
Collector’s signature:
Date and time of collection:
/ /
:
AM
PM
RECEIVING (To be completed by laboratory):
Seal intact?
Yes No
Labels match?
Yes No
Specimen received by: Date received:
3650 Westwind Blvd. Santa Rosa, CA 95403 // Laboratory: 800-255-2159 // Screening Devices: 877-444-0049
Local: 707-577-7959 // Fax: 707-577-0365 // www.redwoodtoxicology.com
11 000 3164 REV4
2-PART FORM 1 of 2 LABORATORY COPY
DONOR CERTIFICATION (To be completed by donor)
I certify that I provided my oral fluid specimen to the collector and that I have not adulterated it in any manner. The specimen was sealed in my presence with a tamper-
evident seal and the information provided on this form and on the label affixed to the specimen tube is correct. I authorize Redwood Toxicology Laboratory to perform
the tests listed and to release the results of this test to:
Donor’s name as listed above (please print): Donor’s ID (SSN OR DL #):
Donor’s signature:
99999 - ANY AGENCY
ORAL FLUID SPECIMEN LABELING
10000 00001
ORAL FLUID TEST REQUISITION
Oral Fluid Drug Test
laboratory test requisition
99999
ANY AGENCY
1111 Anywhere Ln.
Suite 100
Anywhere, CA 95403
Test Panel: 9003 (AMP, COC, m-AMP, OPI, PCP, THC)
GC/MS Confirm:_____________________________________
Donor Initials
Date Collected
Security Seal
REDWOOD TOXICOLOGY LABORATORY
NOTE: Place the center of this
label over the cap of the screening
device or collection device.
10000 00001
LAB BASED COLLECTOR INSTRUCTIONS:
Ensure red cap is tightly fastened. Place the center
of the security seal over the top of the red cap. Be
sure that the barcode runs lengthwise/vertically on
the collection tube.
RTL-Oral Labeling Example
0000000
Security seal
with barcode
APPLY AS SHOWN
Donor initials
Date collected
ON-SITE SCREENING DEVICE EXAMPLE:
Ensure the screw cap is tightly fastened. Write the drug(s) to be
confirmed on the GC/MS confirm line above. Place the center
of the security seal over the top of the cap. Be sure that the
barcode runs lengthwise/vertically on the collection tube.
On-site Screening Device
Security Seal
0000000
Security seal
with barcode
APPLY AS SHOWN
Oral Fluid Test Request Form: Labeling Protocol
How to label your specimens using the oral fluid multi-part forms
LABELING OVERVIEW
The following procedure gives you an easy to follow guide to ensure
your specimens are labeled correctly using the oral uid test request
forms. Correctly labeled specimens will allow the laboratory to pro-
cess your specimens accurately and quickly.
LABELING PROCEDURE
1) Seal container—After the collection process is complete,
ensure the specimen container is tightly sealed.
2) Indicate the test to be performed— Indicate the test to be
performed at the top of the form. For on-site devices, indicate
the drug to be conrmed on the “GC/MS Conrm” line.
3) Sign and date the form— The donor will enter his/her signa-
ture, printed name, date collected and donor ID (SSN or DL#
if required by agency). The collector will verify the information
provided by the donor and validate that the specimen was col-
lected correctly.
4) Place security seal on the collection tube—Once the speci-
men has been veried by the donor and collector, the collector
will remove the security seal from the form and place it across
the top of the collection tube. NOTE: The barcode and number
are required for processing the oral uid specimen. Ensure that
the barcode and number are unobscured and clearly visible on
the side of the collection tube.
5) Package for shipping—After labeling and sealing the speci-
men tightly, place it into a RTL branded plastic baggie with
absorbent material. Ensure baggie is sealed. Store in a
secure area until the specimen is ready to be shipped to
the laboratory.
00000 00000
Donor signature and collector signature lines.
Security seal placed across the collection
tubes cap. Ensure barcode is clearly visible
from side of tube.
00000 00000
Place oral uid specimen into the plastic baggie.
IMPORTANT LABELING INFORMATION
The oral uid sample cannot be processed without the
information supplied on the test request form. If the test
request form does not accompany the specimen, testing
will be delayed.
specimen collection
30
Packaging Protocol: Shipping to Lab
Urine & oral fluid specimen shipping instructions
URINE & SALIVA SHIPPING PRINCIPLES
The following is a brief description of how to label and send your
urine specimen in to the laboratory for testing:
All on-site device samples & lab
urine samples: After labeling and
sealing the specimen tightly, place it
into a RTL branded plastic baggie with
absorbent material. Ensure baggie is
sealed. Store in a secure area until the
specimen is ready to be shipped to
the laboratory.
Lab oral uid samples: After labeling
and sealing the specimen tightly, place
the transport tube into the original outer
packaging provided with the collector
device. Ensure baggie is sealed. Store
in a secure area until the specimen is
ready to be shipped to the laboratory.
Oral uid samples must be received by
the lab within 7 days of collection. Speci-
mens should be refrigerated until shipped.
The test request form (chain of custody form) must be placed in
the same shipping bag as the specimen. If the form is sent in a
separate bag, it will not be matched to the specimen.
SPECIMEN SHIPPING
Your account has been set up with one of the following specimen
shipping methods:
EXPRESS DELIVERY SERVICE—5 OR MORE SPECIMENS
Please use the packaging materials and labels that are included with
your lab supplies. Instructions for packing and shipping specimens
will be enclosed with your supply shipment.
U.S. POSTAL SERVICE—LESS THAN 5 SPECIMENS
Security
Seal
Place urine specimen into
the plastic baggie.
Place saliva specimen into
the plastic baggie.
5 or more specimens: Use express delivery package
Security
Seal
Security
Seal
Security
Seal
Security
Seal
Security
Seal
LABORATORY SHIPPING PACK
Supplied by RTL
Fewer than 5 specimens: Use USPS Pre-paid Mailer Box
White Oral Fluid Box
Security
Seal
Security
Seal
Brown Urine Box
Please use the pre-paid U.S. mailer boxes. Do not mix oral uid
and urine specimens in the same postage-paid mailer box! Per U.S.
Postal Service regulations, use the box provided specically for
each type of test as indicated on the pre-paid label.
SHIPPING/PICKUP
If you need help with scheduling an express delivery pick-up,
please call Toxicology Support Services at 800.255.2159.
(See page 57 for additional information.)
Supplies Re-ordering: Lab supply re-ordering is available to existing
clients with an account number. RTL offers two convenient methods for
re-ordering drug testing supplies:
Email: supplies@redwoodtoxicology.com
Web: https://www.redwoodtoxicology.com/resources/supply_form
Please order supplies through RTL ONLY. Do not ask drivers for labels or bags since
RTL provides its clients with custom supplies. The supplies have specialized routing
information that drivers do not have access to. This information is also important for
billing purposes.

REFERENCE GUIDE // Specimen collection
31
IMPORTANT PACKAGING NOTICE
FEDERAL LAW REQUIRES THAT SPECIMENS BE PACKAGED
IN BAGS WITH ABSORBENT MATERIAL.
PLEASE BE SURE TO FASTEN THE LID OF THE BOTTLE
TIGHTLY. LEAVE THE ABSORBENT MATERIAL IN THE BAG
WITH THE SPECIMEN BOTTLE.
LEAKY SPECIMENS MAY BE RETURNED TO YOUR AGENCY
BY YOUR SHIPPING PROVIDER.
IF YOU REQUIRE TRAINING ON SPECIMEN COLLECTION
PROCEDURES, PLEASE CONTACT RTL’S CLIENT SERVICES
DEPARTMENT:
Phone: 800.255.2159
Fax: 707.577.0365
Email: clientservices@redwoodtoxicology.com
THANK YOU.


33
Result reporting &
collection management
Redwood Toxicology Laboratory’s objective is to provide its clients with all the tools possible
to help deter and prevent drug abuse. Reliable and useful toxicology reports are essential to
this objective. Toxicology results are delivered in the following formats:
Web-Result Reporting & Collection Management—RTL’s ToxAccess
™
site
provides a secure and complete solution for tracking, managing and printing your drug test
reports and chain of custody forms.
• Fast collections—print chain of custody forms using a simple, step by step process
on a single screen
• Accurate data—no more hand-written labels and laboratory data entry errors
• Real-time tracking—track specimens from collection to reporting; includes COC
scans with donor, collector and lab receiving signatures
• Program management—schedule tests, generate collection rosters and
view no-shows
• Powerful reporting—pending specimens, drug statistics, donor summaries
and more
Mail/Fax Reports—RTL offers reporting solutions via U.S. mail or facsimile. Please
indicate your preferred method at the time of account set-up.
REFERENCE GUIDE // Result reporting

result reporting
34
RTL’s reporting website provides a secure and convenient solution
for viewing, managing and printing your toxicology results. We have
expanded the collection features of ToxAccess
™
to create a more
comprehensive drug test management solution for our clients.
These productivity enhancements offer the following features,
advantages and benets:
FEATURES
FAST COLLECTIONS. Now your drug test collections can be
performed quicker, easier and more efciently because collections
are performed on a single screen in just a few simple steps. All
donor information is saved and stored in the website so you only
have to enter it once. (With traditional chain of custody forms you
have to ll out the donor information time and time again.)
CLEAR, ACCURATE DATA . The information you input is
automatically transferred into RTL’s laboratory information system,
eliminating both the errors caused by hand-written labels and
laboratory data entry errors.
COMPLETE, REAL-TIME TRACKING. Track specimens
every step of the way—from collection to reporting—in real time,
24/7. These steps include:
• Scheduled for testing—schedule donor groups for testing on
a particular date (Optional)
• Collected—the specimen has been collected
• Shipped—the specimen has been shipped to the lab; you
can even enter your FedEx tracking number to track the
shipment (Optional)
• Received by lab—the specimen has been received by the lab
and is being tested
• COC scans—view, print and manage signed COC’s
• Reported—the specimen has been reported
CONVENIENT, IN-CONTROL PROGRAM MANAGEMENT.
Get the big picture by organizing donors into specic groups and then
schedule them for testing utilizing a monthly calendar. View groups
scheduled for each day, the collection roster for the current date,
and no-show lists.
POWERFUL, USABLE REPORTING. Generate, and within
seconds, put at your ngertips a complete listing of a donor’s test
results, pending specimens, drug statistics, no shows and more.
TOTAL, DIGITAL DATA COLLECTION. ToxAccess
™
is a complete
donor data management solution that captures all the following infor-
mation on each donor and stores it electronically:
• Name (First, M.I., Last) • Birth Date
• Sex • Intake Date
• Counselor/Probation Ofcer • Location/Agency
• Default Test/Panel • Donor Group
• Status (Active/Inactive) • Special Instructions
• Unique Identier (e.g. SSN, Employee ID)
ToxAccess
™
Result Reporting & Collection Management
Figure 1: Result Summary &
Specimen Search
View more information at: www.redwoodtoxioclogy.com.
To request access, training or support please contact:
Information Technology Department (IT)
Phone: 800.255.2159, ext. 34311
Email: helpdesk@redwoodtoxicology.com

35
REFERENCE GUIDE // Result reporting
Sample Final Toxicology Report
Standard Result Reporting
Toxicology results
LC/MS/MS
LC/MS/MS
LC/MS/MS
LC/MS/MS
GC-FID - Gas Chromatography - Flame Ionization Detector
GC/MS - Gas Chromatography / Mass Spectrometry
FINAL REPORT
Standard reporting from Redwood Toxicology Laboratory includes
toxicology reports which are delivered via fax, mail or the internet
(ToxAccess
™
). The combination of RTL’s leading testing methods and
chain of custody documentation guarantees legal defensibility.
For more information on specic drugs, including; classications,
metabolism, general abuse information and methods of analysis,
please refer to the Drug Information section, beginning on page 37.
For more information on how to read creatinine levels, please see
page 56 within the Drug Information section.
FINAL REPORT OVERVIEW
1) Donor and agency information—Contains client, donor/
patient and collection information.
2) Final result summary—Features immediate result status,
including a list of any detected analytes or if “None detected.”
3) Test ordered—Lists the tests that were ordered by the client,
and features test panel code information.
4) Drug test overview—Shows test results for each drug, test
methodology and cutoff.
5) Specimen comments—Comments may accompany labora-
tory results and ndings.
1
2
4
5
3


37
Drug information
Included herein is specic drug information including classications, metabolism,
general abuse information and methods of analysis.
This section will assist you in understanding specic drugs of abuse and how
Redwood Toxicology Laboratory’s wide range of urine and oral uid screening
programs test for each drug.
REFERENCE GUIDE // Drug information
drug information
38
CLASSIFICATION
Common alcohols are a group of compounds whose structures
include a hydroxyl group attached to a carbon chain of varying
length. The number of carbons determine both the name and indi-
vidual properties. The common alcohols are designated as primary,
secondary, or tertiary depending on the number of carbons that are
attached to the carbon bearing the hydroxyl group. Examples of
primary alcohols include methanol and ethanol, which contain one
and two carbons respectively. Secondary alcohols include isopropa-
nol, a three carbon alcohol. Tertiary or four carbon alcohols include
N-butanol. The information contained herein will deal primarily with
ethanol, or beverage alcohol.
METABOLISM
Ethanol is a small molecule which is readily soluble in water and
penetrates membranes throughout the GI tract, including the mouth,
stomach and small intestine. Rate of absorption varies greatly due
to factors such as type of beverage ingested, quantity of food in the
stomach, frequency of gastric emptying, drinking pattern, etc. The
average time to peak absorption is usually 30 to 60 minutes, but
may range from 15 minutes to 3 hours. Approximately 95% of an
ingested dose of ethanol
is metabolized by liver
enzymes, principally alco-
hol dehydrogenase, with
the remainder eliminated
unchanged in the urine,
breath, sweat, and feces.
Ethanol is metabolized to
acetaldehyde and then to a nal end product of acetic acid. Distribu-
tion of alcohol throughout the body occurs via the blood supply and
since ethanol is hydrophilic (strong afnity for water), it will diffuse
into body tissue or uid compartments such as urine, saliva, plasma,
etc. Since plasma or serum have a higher percentage of water by
unit volume, it will have a higher ethanol concentration than whole
blood. A wide range of elimination rates exists, however, ethanol is
typically metabolized at approximately .015-.018 gm/dL per hour in
healthy individuals. Therefore, the detection time of ethanol in body
uids is dose dependent.
ABUSE
Ethanol is the most widely consumed drug in society and is
generally consumed socially. Ethanol is predominantly consumed
as fermented or distilled beverages and is also a component of
mouthwashes, medicinal, and industrial products. Fermented
beverages such as beers and ales contain 3-6% ethanol by volume,
wines contain 10-12%, and distilled spirits contain 20-60% ethanol.
Acute ingestion of ethanol typically leads to progressive stages of
effects depending upon the amount consumed. With low to moder-
ate consumption, a person may initially experience mild euphoria,
sociability, decreased inhibitions and the beginning of sensory-motor
impairment. With increased consumption the effects can progress
to emotional instability, loss of perception, memory and comprehen-
sion in addition to decreased response time and slurred speech,
staggered gait and loss of muscular coordination. Finally, excessive
acute ethanol consumption can lead to impaired consciousness,
respiratory depression, coma and death. Chronic ethanol abuse can
result in multi-organ pathological effects. These can include cirrho-
sis of the liver, acute and chronic gastritis, pancreatitis, cardiomy-
opathy and various neurological and metabolic disorders.
METHODS OF ANALYSIS
One of the most widely used techniques for detecting alcohols
in urine include enzyme assay utilizing alcohol dehydrogenase.
The method is very sensitive and specic in that methanol and
acetone are not detected. However, longer chain alcohols such as
isopropanol may be detected. This is appropriate for clinical set-
tings, however, for forensic purposes, a secondary more denitive
conrmation method is required. Gas chromatographic methods,
either direct injection or headspace sampling, with the incorporation
of an internal standard offer very accurate and precise quantitation
of ethyl alcohol, in addition to identifying other volatile compounds
such as methanol, acetone, isopropanol. Denitive identication of
ethanol and accurate quantitation are required when relating a blood
alcohol concentration to a particular level of impairment. This is
especially critical when the blood alcohol concentration is to be used
as evidence to determine whether grounds exist for presumption
of impairment.
H
3
C
CH
2
OH
Alcohol
Drug information
REFERENCE GUIDE // Drug information
39
CLASSIFICATION
Amphetamine and methamphetamine are Schedule II drugs included
in a group of chemicals called sympathomimetic amines, which
contain a phenethylamine chemical nucleus. Sympathomimetic
amines mimic the effects of the endogenous neurotransmitters such
as epinephrine (adrenaline), norepinephrine (noradrenaline) and
dopamine. Also included in this group are various over-the-counter
drugs such as phenylpropanolamine, pseudoephedrine, ephedrine
as well as the Schedule I drug methylenedioxymethamphetamine
(MDMA or Ecstasy). The amphetamines are powerful central
nervous system stimulants and can be taken orally, intravenously,
snorted or smoked. Methamphetamine is one of the most commonly
abused drugs in the Western United States. It is readily synthesized,
with ephedrine being used as the primary precursor.
METABOLISM
Amphetamines are rapidly absorbed from the gastrointestinal
tract and are either deactivated by the liver or excreted unchanged
into the urine. Methamphetamine is excreted primarily unchanged
(44%) and some of the drug (6%) is metabolized and excreted as
amphetamine. Amphetamine is also excreted largely unchanged
(30%) with 20-25% being metabolized to deaminated (hippuric and
benzoic acids) and hydroxylated metabolites. The elimination rate of
amphetamines varies with the pH of the urine, as at low pH the
excretion of unchanged drug increases, while at high pH the excre-
tion of unchanged drug decreases. Within a few hours after any
type of administration, amphetamines appear in the urine and can
typically be detected for up to 72-96 hours.
ABUSE
Amphetamines, particularly methamphetamine, are among the
most popular drugs of abuse. Common street names include speed,
crank, crystal, meth, and ice. Ice and crystal meth are crystals
of methamphetamine HCL. Snot and glue are oils formed from
methamphetamine free base and baking soda. Methamphetamine is
frequently smoked in a glass pipe as it is easily volatilized into a gas
that is inhaled. Although the ice form is primarily found in Hawaii and
the western United States, it has gained the most notoriety mainly
due to the fact that it is >90% pure methamphetamine HCL and its
effects are rapid, intense, and of longer duration than other forms of
methamphetamine.
The signs and symptoms
associated with the abuse
of methamphetamine
depend upon the amount
used and the duration of
use. With infrequent or
low dose use, a person
may experience euphoria,
lowered anxiety, talkativeness, decreased appetite, increased sexual
arousal, increased alertness, and decreased fatigue. Physiologically
there can be increased heart rate and blood pressure. With increased
dose or prolonged abuse (either binge or chronic), an individual may
experience a set of secondary effects that can include increased
anxiety, irritability, aggressiveness, paranoia and hypersexuality.
Physiological effects can include dilated pupils, dry mouth, hippus,
increased body temperature and tachycardia. In overdose situa-
tions, a person may experience hallucinations, coma or death. Crash
symptoms typically follow binge abuse of methamphetamine. This
phase is marked by extreme fatigue, depression, mental exhaustion
and prolonged periods of sleep.
METHODS OF ANALYSIS
Immunoassays are common methods for detecting amphetamines
in urine. Enzyme immunoassay (EIA) is the most commonly used
immunoassay that detects both methamphetamine and amphet-
amine to varying degrees of sensitivity and specicity. However,
it will cross-react with several over-the-counter cold and diet
preparations which indicates the importance of conrmatory test-
ing for samples screened presumptively positive by immunoassay
tests. Gas chromatography/mass spectrometry (GC/MS) and liquid
chromatography/tandem mass spectrometry (LC/MS/MS) provides
reliably sensitive and specic solutions as conrmatory methods.
CH
2
CH
3
CH
N
H
CH
3
Amphetamines
Drug information
drug information
40
CLASSIFICATION
Barbiturates are a class of drugs capable of producing CNS
depression, and depending upon the drug and dosage, may produce
varying states of sedation or hypnosis and are thus classied as
sedative/hypnotics. They are further categorized according to the
duration of their effects, ranging from ultra-short acting, short act-
ing, intermediate acting, and long acting. Duration of effects lasts
anywhere from 15 minutes for the ultra-short acting barbiturates to
a day or more for the long acting drugs. Short and intermediate act-
ing barbiturates include amobarbital, butalbital, pentobarbital, and
secobarbital, while the long acting barbiturates include phenobarbi-
tal. Other common therapeutic indications for use are as anticonvul-
sants and for migraine headaches.
METABOLISM
Barbiturates are distributed throughout the body with highest
concentrations occurring in the brain, liver and kidneys. In general,
duration of action is dependent upon lipid solubility and extent of
protein binding with the short acting barbiturates showing the most
lipid solubility and percentage of protein binding. The short and
intermediate acting
barbiturates are nearly
entirely metabolized by
the liver and excreted
in the urine, while 25-
50% of a dose of a long
acting barbiturate is
excreted as unchanged
drug. The half-life is variable with short acting barbiturates being
detectable in urine for 24 hours and the long acting drugs detectable
for 2-3 weeks following ingestion.
ABUSE
The most common detected barbiturates are butalbital and pheno-
barbital. Butalbital is routinely prescribed for migraine and muscle
relaxation while phenobarbital is primarily prescribed for seizure
disorders. Trade and street names of some common barbiturates
are as indicated below.
Chemical Name Trade Name Street Name
Amobarbital Amytal Yellow Jackets
Butalbital Fiorinal Blue Devils
Secobarbital Seconal Reds
Phenobarbital Luminal Downers, Goofballs
Chronic abuse leads to tolerance, and abrupt discontinuance of use
can induce a life-threatening withdrawal syndrome that can result
in seizures.
METHODS OF ANALYSIS
Immunoassays readily detect barbiturates as a class of drugs.
Specic barbiturate identication can be accomplished by
utilizing conrmatory methods such as gas chromatography/
mass spectrometry (GC/MS) and liquid chromatography/tandem
mass spectrometry (LC/MS/MS).
NH
NH
CH
3
CH
2
Barbiturates
Drug information
REFERENCE GUIDE // Drug information
41
CLASSIFICATION
The benzodiazepines are a class of drugs primarily classied as anti-
anxiety, sedatives, or hypnotics. All contain a benzene ring fused to a
7-membered diazepine ring, hence the term benzodiazepine. Various
modications and substitutions of the ring structure yield compounds
of similar activities. The clinical effects of these drugs result from
actions on the central nervous system and these effects include
sedation, hypnosis, muscle relaxation, and anticonvulsant activity.
METABOLISM
The benzodiazepines are well absorbed after oral administration and
are rapidly distributed throughout the body. They are extensively
metabolized by the liver, and in general, slowly excreted in the urine
as pharmacologically inactive conjugated metabolites. Some metabo-
lites may possess some pharmacological activity of their own, thus
displaying the “next day” effects of some benzodiazepines. Oxazepam
is a common urinary metabolite of several benzodiazepines such as
diazepam and temazepam. Duration of detectability in urine is varied.
Ingestion of therapeutic dosages may be detectable for 1-3 days
while extended usage over a period of months or years can extend
excretion times up to 4-6 weeks after cessation of use (depends on
dosage & benzodiazepine).
ABUSE
The benzodiazepines are considered one of the most widely pre-
scribed drugs in the United States, thus leading to its widespread
abuse. Diazepam (Valium
®
) and alprazolam (Xanax
®
) are two of the
most widely abused of the benzodiazepines. Many abusers will
attempt to accentuate the effects of benzodiazepines by the
concomitant use of alcohol or other CNS depressant drugs. As a
result, benzodiazepines are involved in approximately one third of all
drug self induced poisonings. Other commonly abused benzodiaz-
epines are chlordiazepoxide, urazepam, clonazepam, and lorazepam.
Prolonged high doses of benzodiazepines can cause dependency and
a withdrawal syndrome may occur following abrupt cessation of use.
METHODS OF ANALYSIS
There are many problems associated with a comprehensive approach
to the analysis of the benzodiazepines. The benzodiazepines are a
very diverse and complex group of compounds which are extensively
metabolized in urine. For this reason, it is not always possible to
determine the parent drug with urine testing. In addition, dosage
levels and half-life varies substantially between the benzodiazepines
affecting the ability to detect therapeutic use of some benzodi-
azepines. Therefore, the
analytical detection limits
may preclude the detection
of therapeutic use.
The most common analytical
methods to screen for the
presence of benzodiazepines
in urine are the immunoas-
say methods such as enzyme immunoassay (EIA). The immunoassay
methods are class specic in that they detect oxazepam, a common
metabolite of many benzodiazepines. However, many other structur-
ally similar benzodiazepines may also be detected.
While immunoassay cross-reactivity to non-benzodiazepine
compounds is extremely rare, most immunoassay manufacturers
recommend that positive results be conrmed by alternate specic
analytical method such as gas chromatography/mass spectrometry
(GC/MS) or liquid chromatography/tandem mass spectrometry
(LC/MS/MS). Most routine conrmation methods are targeted to
detect the group of benzodiazepines which share a common meta-
bolic pathway and metabolize to nordiazepam and oxazepam thus
making these methods class specic. However, common benzodi-
azepines such as alprazolam (Xanax
®
), lorazepam (Ativan
®
), and
clonazepam (Klonopin
®
) do not share this metabolic pathway and
must be conrmed by specic techniques, such as GC/MS or LC/MS/
MS. It is essential to understand the advantages and limitations of
the various laboratory analytical methods to ensure proper detection
of benzodiazepines. If a case history indicates the use of a particular
benzodiazepine, then a method must be chosen which will have the
necessary sensitivity and specicity to identify the drug of interest.
H
3
C
N
CI
N
Benzodiazepines
Drug information
Chemical and trade names are as follows:
Chemical Name Trade Name
Alprazolam Xanax
®
Chlordiazepoxide Librium
®
Clonazepam Klonopin
®
Clorazepate Tranxene
Diazepam Valium
®
Flunitrazepam Rohypnol
®
Flurazepam Dalmane
®
Lorazepam Ativan
®
Midazolam Versed
®
Oxazepam Serax
®
Prazepam Centrax
®
Temazepam Restoril
®
Triazolam Halcion
®
drug information
42
CLASSIFICATION
Buprenorphine (Suboxone, Subutex) is a semi-synthetic thebaine
derivative that has both analgesic and opioid antagonist proper-
ties. As an analgesic, buprenorphine is approximately 25 to 40
times more potent than morphine and as an opioid antagonist it is
roughly equivalent to naltrexone. Buprenorphine is prescribed in the
hydrochloride form and therapeutic dosages range from 0.3 - 0.6
mg when given parenterally, or 0.2 - 0.4 mg sublingually, every
6 - 8 hours. The use of larger daily doses (2 - 16 mg daily) have
been used successfully for the treatment of opiate withdrawal or
maintenance. Overdose symptoms include confusion, dizziness,
pinpoint pupils, hallucinations, hypotension, respiratory difculty,
seizures and coma. Fatalities due to buprenorphine overdosage
alone and by poly-drug use have been reported.
METABOLISM
Buprenorphine is rapidly metabolized in the liver by the cytochrome
P450 system to form a pharmacologically active N-dealkylated
metabolite, norbuprenorphine and glucuronide conjugates.
Buprenorphine and norbuprenorphine are excreted in urine almost
exclusively as glucuronides with very little free drug being detected.
Studies indicate that concentration of free buprenorphine and norbu-
prenorphine in urine can be less than 1 ng/mL following therapeutic
administration, but can range up to 20 ng/mL in abuse situations.
Total buprenorphine and norbuprenorphine concentrations in urine,
ranging from 0.5 - 2936 ng/mL and 4.0 - 4462 ng/mL respectively,
have been reported following daily doses between 0.2 - 24 mg. The
corresponding median norbuprenorphine to buprenorphine ratio was
0.23. However, there is signicant inter- and intra-individual vari-
ability in the ratio because the elimination kinetics of norbuprenor-
phine are slower; therefore, the ratio is greatly inuenced by dosage
and sample time. Approximately 95% of a labeled dose is excreted
within 144 hours, 68% in the feces and 27% in the urine.
ABUSE
Like methadone when buprenorphine is taken by an individual
who is addicted to heroin or other opioid, buprenorphine reduces
craving and helps the person remain drug-free. Because of its opioid
effects, buprenorphine can also be abused, particularly by individu-
als who are not physically dependent on opioids.
Compared with methadone, buprenorphine has a relatively lower
risk of abuse, dependence, and side effects, and it has a longer
duration of action. Because buprenorphine is a partial opioid ago-
nist, its opioid effects, such as euphoria and respiratory depression,
as well as its side effects, reach a ceiling of maximum effect, unlike
with methadone or heroin. For this reason, buprenorphine may be
safer than methadone, as long as it is not combined with sedatives
such as tranquilizers or alcohol. The side effects of buprenorphine
are similar to those of other opioids and may include nausea, vomit-
ing, and constipation. Both buprenorphine and buprenorphine with
naloxone can result in the opioid withdrawal syndrome if used by
people on high doses of other opioids. Symptoms of opioid with-
drawal can include: dysphoria, nausea and vomiting, muscle aches
and cramps, sweating, tearing, diarrhea, mild fever, running nose,
insomnia, and irritability.
METHODS OF ANALYSIS
Enzyme immunoassay (EIA) is used as a screening method for the
detection of buprenorphine. The assay has equal cross reactivity to
norbuprenorphine, the primary urinary metabolite of buprenorphine.
Conrmation of screened positive urines should be performed by a
specic method such as
gas chromatography-
mass spectrometry (GC/
MS) or liquid chromatog-
raphy-tandem mass spec-
trometry (LC/MS/MS).
Buprenorphine
Drug information
HO
O
OCH
3
H
HO
N
REFERENCE GUIDE // Drug information
43
Cocaine
Drug information
CLASSIFICATION
Cocaine (benzoylmethylecgonine) is a central nervous system
stimulant derived from the leaves of the coca plant. Cocaine has
two major pharmacological actions; one is a local anesthetic, and
the other is an indirect acting sympathomimetic having many of the
properties of an amphetamine. The drug is either in the salt/powder
form (cocaine HCL) which can be administered by snorting or intra-
venous injection or in the free base “crack” form which is smoked.
METABOLISM
After smoking, cocaine is rapidly absorbed with peak plasma
concentrations occurring at about 5 minutes, versus 30-40 minutes
following intranasal ingestion. Cocaine is extensively metabolized
by the liver and blood
enzymes with approxi-
mately one percent of the
dose excreted in the urine
unchanged. The major
metabolite found in the
urine is benzoylecgonine
(25-40% of the dose),
followed by ecgonine methyl ester (18-22%). Depending upon the
dosage ingested, frequency of use, and metabolic variation, benzoy-
lecgonine can remain detectable in the urine for as long as 48-96
hours post ingestion.
ABUSE
Cocaine produces a short-lived, intense high which is extremely
addictive. The signs and symptoms associated with the abuse of
cocaine depend upon the amount used and the duration of use.
With infrequent or low dose use a person may experience euphoria,
lowered anxiety, talkativeness, decreased appetite, increased sexual
arousal, increased alertness, and decreased fatigue. Physiologically
there can be increased heart rate and blood pressure.
With increased dose or prolonged abuse (either binge or chronic)
an individual may experience a set of secondary effects that can
include increased anxiety, irritability, aggressiveness, paranoia and
hypersexuality. Physiological effects can include dilated pupils, dry
mouth, hippus, increased body temperature and tachycardia. In
overdose situations, a person my experience hallucinations, coma or
death. Crash symptoms typically follow binge abuse of cocaine. This
phase is marked by extreme fatigue, depression, mental exhaustion
and prolonged periods of sleep.
METHODS OF ANALYSIS
Enzyme immunoassay (EIA) is a widely used screening method
designed to specically detect benzoylecgonine and to a lesser
extent, cocaine and ecgonine methyl ester (secondary cocaine
metabolite). Redwood Toxicology Laboratory utilizes liquid chroma-
tography/tandem mass spectrometry (LC/MS/MS) for conrmation.
This method offers excellent sensitivity and specicity and is the
method of choice for the conrmation of the immunoassay
positive screens.
H
3
C
H
3
C
C
C
N

drug information
44
CLASSIFICATION
Recreational drugs produced in the laboratory have been around
since at least the middle of the 20th century, when LSD was rst
studied. But over the past few years, a new wave of synthesized
chemicals, so-called “designer drugs” and “designer stimulants”
have had a signicant impact on the drug culture.
The term “designer stimulants” is used for chemicals that produce
similar subjective effects to illegal recreational drugs. Development
of synthesized drugs may involve altering the molecular structure
of existing drugs, or identifying different chemical structures that
generate the same effects that illegal drugs produce.
A group of synthetic compounds consisting of β-keto- and methy-
lendioxy- derivatives of amphetamines and derivatives of piperazine
have been recently developed as recreational (“party”) drugs with
psychoactive properties. They gained popularity as legal alternative
to amphetamines and cocaine and have been abused worldwide,
prompting investigation into their safety. These drugs were proven
to possess central stimulation effects similar to those of other
illicit drugs.
Due to their high potential for abuse and addiction, most designer
stimulants have been recently banned in many European countries,
Australia and New Zealand. A federal ban enacted in July 2012 tar-
gets MDPV and Mephedrone, two designer drug compounds found
in so-called bath salts. This important measure will prove critical in
deterring abuse from these dangerous designer drugs. Nevertheless,
they are still readily available via the Internet and in many “head-
shops” around the country.
METABOLISM
Designer stimulants are excreted in urine unchanged and as
conjugated hydroxy-metabolites. Cathinone is known to metabolize
extensively by reduction of β-keto group into free norephedrine
and nor-ψ-ephedrine. Similar metabolic path is expected for other
synthetic stimulants possessing β-keto structure.
ABUSE
Synthetic designer stimulants are produced in clandestine labora-
tories and are commonly sold as “bath salts” at smoke shops or
available online. They are sold under a variety of names that include
Ivory Wave, Cloud Nine, Bliss, Red Dove, Vanilla Sky and
Hurricane Charlie.
These “bath salts” are in reality potent crystallized chemicals that
may be snorted, swallowed or smoked. They contain powerful
stimulants such as methylone, methylenedioxypyrovalerone (MDPV)
and mephedrone, which mimic the stimulating effects of cocaine,
methamphetamine or MDMA. Additionally, some forms of designer
stimulants may be sold and veiled as MDMA (ecstasy) tablets.
Effects sought by users include feelings of physical and mental well-
being, exhilaration, euphoria, increased alertness, elevated motor
activity, and postponement of hunger or fatigue. Young adults in the
United States and other countries have reportedly died from using
these products. While synthetic stimulants appear to affect users
in ways similar to amphetamines and cocaine, reports concerning
aggression, tachycardia, paranoia and suicide suggest that they
may be more acutely toxic. There are no known medical uses for
synthetic stimulants, and long-term effects are unknown, although
experts have stated that cardiovascular effects can last for days
after ingestion.
Several agencies have issued alerts about synthetic stimulants,
noting ease-of-access concerns and the number of nationwide
emergency-room visits related to these drugs.
In 2011, poison centers nationwide responded to over 6,000 calls
related to bath salts. In 2012, that number dropped to over 2,500
and in 2013 the number of calls was over 900 showing that the true
extent of the public health threat is still prevalent.
METHODS OF ANALYSIS
RTL’s test utilizes gas chromatography/mass spectrometry (GC/
MS) or liquid chromatography/tandem mass spectrometry (LC/MS/
MS) for screening and conrmation of designer amphetamines,
cathinones and designer piperazines. All presumptive positive
specimens are conrmed using a second aliquot prior to reporting
positive results. The analytical methods used by RTL are scienti-
cally accepted.
Two test panel variations are available: an expanded designer
stimulant panel covering 21 drugs or the limited panel covering
MDPV, Methylone and Mephedrone.
Designer Stimulants
Drug information

REFERENCE GUIDE // Drug information
45
CLASSIFICATION
Diuretics elevate the rate of bodily urine excretion (diuresis). All
diuretics increase the excretion of water from the body, although
each class of diuretics does so in a distinct way employing different
mechanisms of action. In medicine, diuretics are used to treat heart
failure, liver cirrhosis, hypertension and certain kidney diseases.
Side effects include dehydration, hypotension, disturbed electrolyte
balance, muscle cramps and weakness.
Diuretics are prescription drugs with relatively low potential for
abuse among the general population. They are not classied as
Controlled Substances in the United States. There are several
categories of diuretics:
• High ceiling loop diuretics such as furosemide, bumethanide
and ethacrynic acid may cause substantial diuresis. They
inhibit the body’s ability to reabsorb sodium in the kidney
which leads to retention of water in the urine as water nor-
mally follows sodium back into the extracellular uid.
• Thiazides such as hydrochlorothiazide, cause moderate
diuresis. They act as carbonic anhydrase inhibitors with the
major site of action in the renal distal tube. They enhance
excretion of sodium, potassium, calcium and chlorine ions.
• Potassium sparing diuretics such as amiloride, triamterene
and spironolactone do not promote the secretion of potas-
sium into the urine; thus potassium is spared and not lost as
much as in other diuretics.
• Osmotic diuretics like mannitol, glucose and other sugars are
ltered in the kidneys, but cannot be reabsorbed leading to
elevated water elimination with urine.
• Low ceiling diuretics—the term is used to indicate a
pharmacological prole with rapidly attening dose
effect curve (in contrast to “high ceiling”, where the
relationship is close to linear). The thiazides usually
fall into this category.
METABOLISM
Most diuretics are excreted in urine with minimal metabolites. A
few exceptions include spironolactone metabolizing into canrenone
and canrenoic acid; and triamterene, which in the body undergoes
conversion to hydroxytriamterene sulfate.
ABUSE
Diuretics are sometimes abused by people with eating disorders for
weight loss. Use of diuretics in sports is prohibited. Increased urine
ow would reduce concentrations of banned performance enhanc-
ing substances in urine such as anabolic steroids, thus complicating
their detection in doping control (masking). In sports where weight
categories are involved diuretics are abused as weight reducing
agents. Diuretic abuse in sports is unethical and dangerous for ath-
lete health (dehydration). Diuretic testing is a part of routine doping
control in sports.
METHODS OF ANALYSIS
RTL utilizes Methylation and gas chromatography/mass spectrome-
try (GC/MS), which has been traditionally used for diuretic screening
and conrmation in athletic doping control.
Diuretics
Drug information
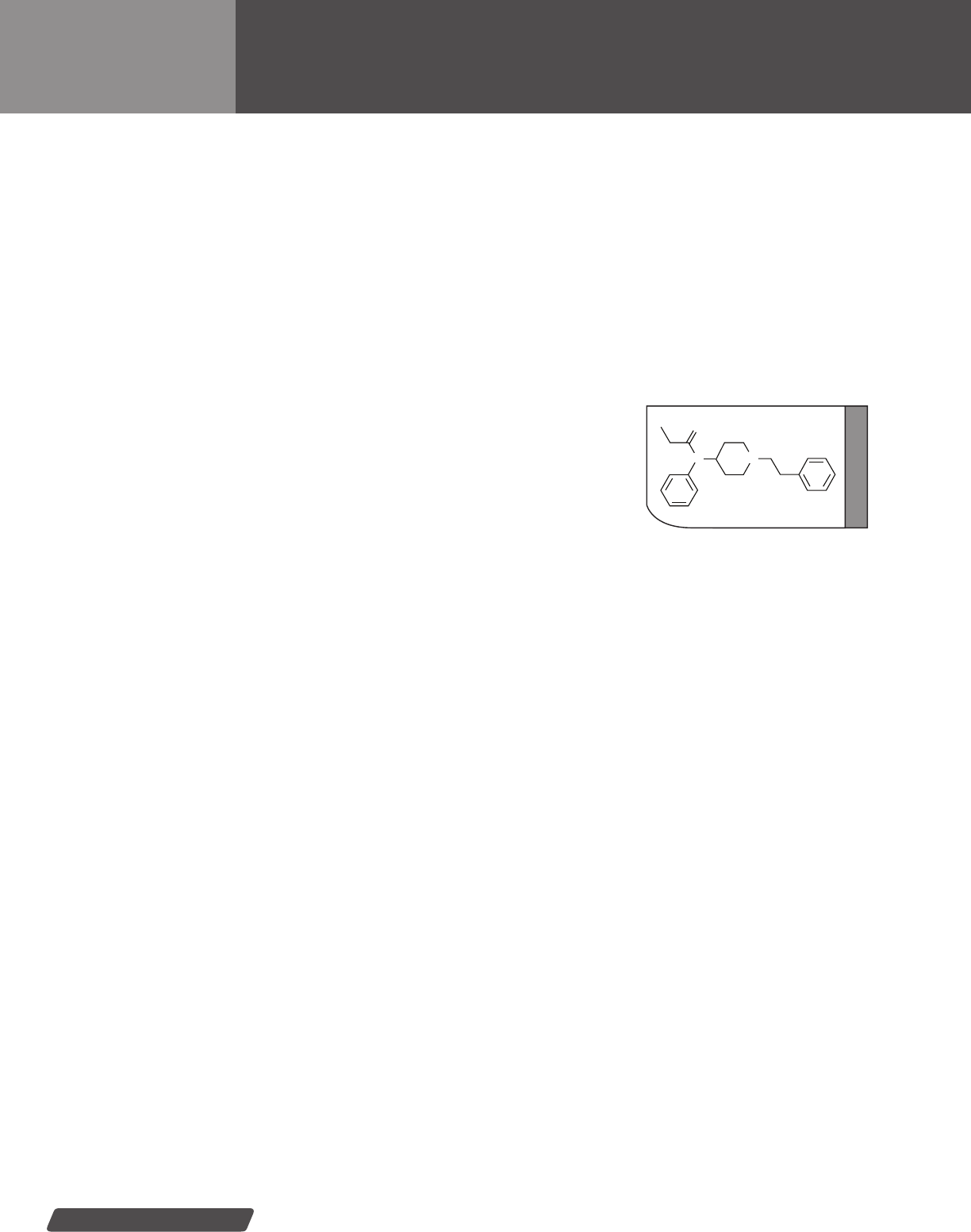
drug information
46
Fentanyl
Drug information
CLASSIFICATION
Fentanyl (Duragesic, Sublimaze, “China White”) is an extremely fast-
acting synthetic narcotic analgesic, of high potency (approximately
100 – 200 times that of morphine) and short duration of action.
There are several analogues and derivatives of fentanyl which are
also abused, and may have higher potencies. Pharmaceutical fen-
tanyl has been available since 1963 as an anaesthetic supplement,
and is available as a citrate salt for I.V or I.M injection. Transder-
mal patches are also available for management of chronic pain or
for breakthrough cancer pain. Fentanyl abuse among healthcare
workers has become popular due to the drugs euphoric effects and
easy availability. Due to the lipophilicity of the drug, fentanyl rapidly
crosses the blood-brain barrier, producing fast and pronounced CNS
effect, such as a heightened euphoria and respiratory depression,
and possible toxic effects which include muscle rigidity, seizures,
coma, and hypotension. Fentanyl also has similar tolerance and
physical dependence properties to those of morphine.
METABOLISM
Fentanyl is rapidly metabolized by the liver to the inactive
metabolites, norfentanyl, hydroxyfentanyl, and hydroxynorfentanyl.
Approximately 85% of an intravenous dose is excreted in urine over
a 3 – 4 day period, with 0.4 – 6% of the drug excreted unchanged,
26 – 55% excreted as norfentanyl, and unknown amounts of
hydroxyfentanyl, and hydroxynorfentanyl excreted.
Fentanyl is administered I.V. or I.M. at single dosage levels of 25 –
100 µg as needed, transdermally at dosages of 25 – 100 µg/hr for
72 hours for chronic pain management, or by oral transmucosal
dosages of 200 – 1600 µg for breakthrough cancer pain. Following
a single 50 – 100 µg fentanyl dose, fentanyl was detected in the
urine of 3 of 7 patients for 24 hours. Urine fentanyl concentrations
ranged from 89 – 449 ng/mL in 4 adults who died following the
excessive use of transdermal fentanyl. In another series of 7 adult
deaths, fentanyl concentrations ranging from 5.0 – 93 ng/mL were
found following self-administered intravenous injections.
ABUSE
Illicit fentanyl which appears on the street in the U.S., is principally
in the form of the transdermal patches, which can be cut up and
eaten, or the gel can be extracted from the patches and smoked
or injected by addicts. Illicitly synthesized fentanyl powder manu-
factured in Mexico has
appeared in the U.S.
recently, and may be
abused by itself or mixed
with heroin or cocaine,
which have resulted in
several deaths.
METHOD OF ANALYSIS
Immunoassay screens are commercially available for detecting
fentanyl in urine specimens. Conrmation of the presumptive posi-
tives is generally performed using a specic technique such as gas
chromatography/mass spectrometry (GC/MS) or liquid chromatogra-
phy/tandem mass spectrometry (LC/MS/MS). Redwood Toxicology
Laboratory utilizes a direct method for the detection of fentanyl in
urine using liquid-liquid extraction, deuterated internal standards,
and GC/MS detection.
O
N
N
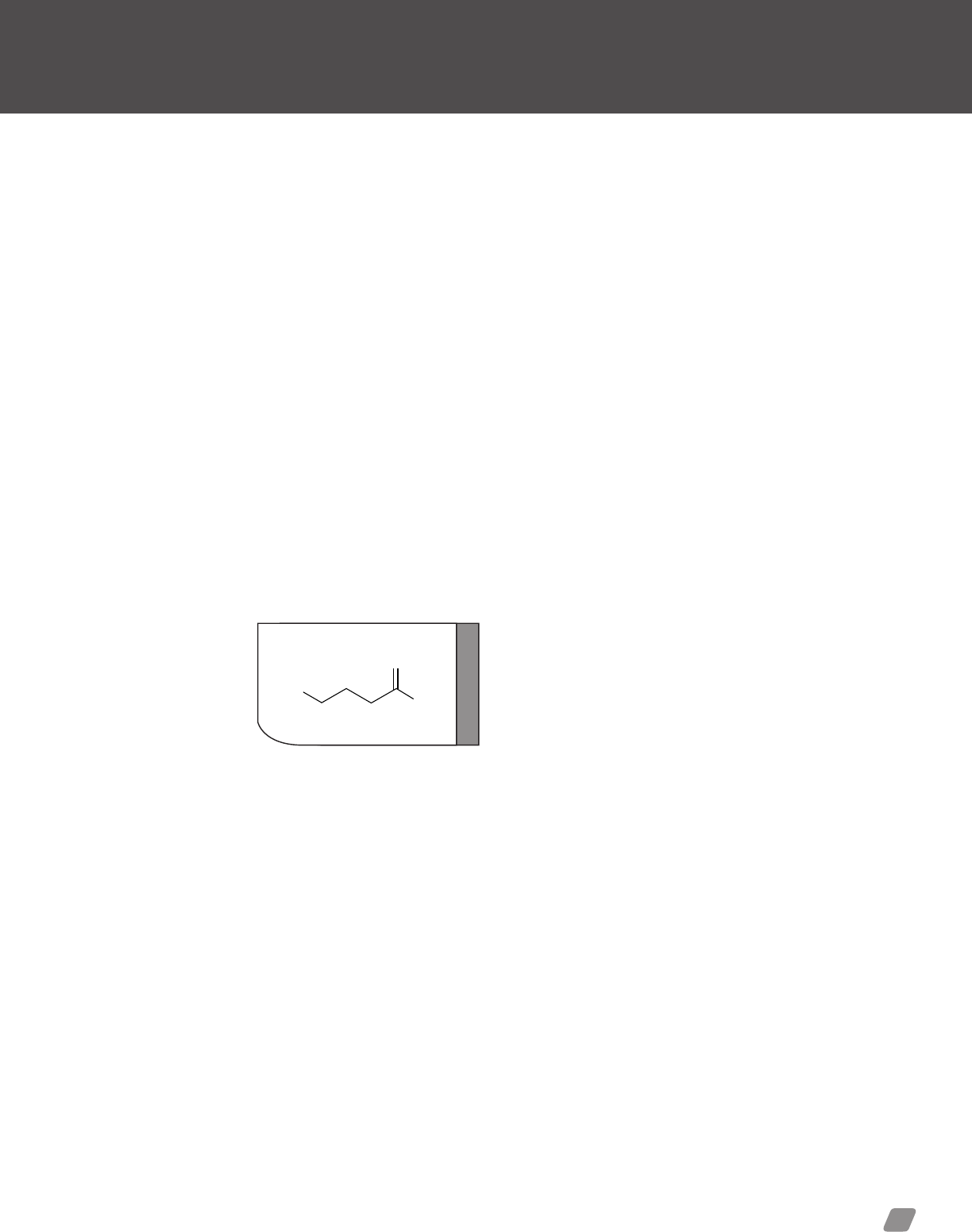
REFERENCE GUIDE // Drug information
47
CLASSIFICATION
GHB was rst developed as an anesthetic, but was discontinued for
this use due to its adverse and unpredictable effects. However, GHB
is readily available and used in Europe as a sedative. The salt form
of GHB has been marketed in gymnasiums and health food stores
for the past few years as a steroid alternative for body-building and
as a tryptophan replacement for sedation. There have also been
increasing reports of GHB being used recreationally as a euphoriant
at “rave” type parties. As typically follows, there have also been
reports of GHB being associated with sexual assault or as a “date
rape” drug due to its severe hypnotic and sedative effect at
higher dosages.
METABOLISM
GHB is thought to be extensively metabolized by alcohol dehy-
drogenase and/or succinic semialdehyde dehydrogenase. Meta-
bolic precursors to GHB,
gamma-butyrolactone
(GBL) and 1,4 butanediol
are also readily available
as substances of abuse.
Endogenous GHB is also a
product of GABA metabo-
lism, and concentrations
of 0 – 6.6 mg/L have been reported. Oral doses of approximately
2.5 g (I teaspoon of GHB powder) dissolved in water, produced
urine GHB concentrations of 29 mg/L in a 100 kg man. Studies also
indicate peak urine GHB concentrations of 100 mg/L following a 100
mg/kg oral dose, and no detectable drug in the urine by 12 hours.
Less than 5% of an oral dose is eliminated unchanged in urine. To
distinguish between endogenous and exogenous GHB, a reporting
cutoff of 10 µg/mL is suggested.
ABUSE
Typical illicit use of GHB involves dissolving 2 – 3 grams of powder
in water or other beverages. Onset of effects occur within 10 – 30
minutes of ingestion and include euphoria, increased libido, drowsi-
ness, reduced inhibitions, dizziness, nausea and may persist for 2
- 5 hours. Toxic effects include hypotension, respiratory depression,
seizure, unconsciousness, and coma. Deaths have been reported
when GHB is used alone or in conjunction with ethanol, heroin, or
ketamine. The California Department of Health Services and the
Food and Drug Administration (FDA) banned over-the-counter sales
of GHB in 1990 and GHB is currently classied as Schedule I by the
federal Controlled Substances Act.
METHOD OF ANALYSIS
Determination of gamma-hydroxybutyric acid (GHB), also known
as liquid Ecstasy, in human samples is challenging. Due to the
small size of GHB, immunological testing is very difcult and limited
immunoassays are available for screening. GHB detection in urine
relies on specic chromatographic methods such as gas chromatog-
raphy/mass spectrometry (GC/MS) or liquid chromatography/tandem
mass spectrometry (LC/MS/MS). RTL’s test utilizes GC/MS for the
direct analysis of GHB from urine after liquid-liquid extraction and
silyl-derivatization. Compared to existing methods, this method
is superior because it is specic to GHB without conversion
to gamma-butyrolactone (GBL).
Gamma-hydroxybutyric Acid (GHB)
Drug information
HO
OH
O

drug information
48
CLASSIFICATION
Marijuana is a preparation derived from the leaves and owering
tops of cannabis plants (Cannabis sativa) that is capable of produc-
ing psychoactive effects when ingested. One of the primary classes
of compounds found in marijuana is called cannabinoids. There are
up to 60 cannabinoids in marijuana with delta-9-tetrahydrocannabi-
nol (THC) being the primary psychoactive constituent.
METABOLISM
When marijuana is smoked, THC is rapidly absorbed through the
lungs and enters the bloodstream in minutes. Following oral inges-
tion, THC does not reach the bloodstream for approximately 1.5-3
hours. Once in the blood, THC is bound to blood proteins and carried
throughout the body where it is either absorbed into body tissues
(including the brain, heart, and fat) or transformed by the liver into
the water soluble metabolites 11-hydroxy-THC and carboxy-THC.
These water soluble metabolites, are readily excreted into the urine,
with the inactive metabolite carboxy-THC being the predominant
metabolite detected. Initially, THC is quickly absorbed into the body
tissues and then is slowly released back into the blood stream where
it is carried to the liver and metabolized. Because THC tends to be
stored in fatty tissues, it accumulates faster than it can be eliminated
in chronic repetitive smokers. This leads to extended retention of
THC which is then eliminated from the body at a relatively constant
rate with an average elimination half-life being estimated at 2-4
days. Urinary concentrations of THC are very difcult to interpret due
to variables such as dosage of THC ingested, frequency of use, tim-
ing of urine collection relative to last exposure to marijuana, rate of
release of stored cannabinoids in adipose tissue, and an individual’s
hydration state. Therefore, the detection of THC metabolites in the
urine is only an indication of past marijuana use and is not related to
the degree of intoxication or impairment.
ABUSE
The psychological effects of THC include an increased sense of well
being or euphoria, relaxation, slowed psycho-motor response, an
altered sense of time, short term memory impairment and impair-
ment of multi-tasking performance.
THC Retention Time
• Infrequent (less than twice/week) Smoking: When screening
assays of 50 ng/mL or greater are used, urine samples will
generally be positive for 1-3 days.
• Regular (several times per week) Smoking: May result in
urine specimens testing positive for 7-21 days.
• Chronic (daily) Smoking: An individual who smokes mari-
juana daily for prolonged periods of time can test positive for
30 days or longer.
• Oral Ingestion: Metabolic proles in urine samples cannot
generally differentiate between marijuana ingested orally ver-
sus marijuana ingested by smoking. However, oral ingestion
requires approximately three times more THC than smok-
ing to produce similar effects or “highs”; therefore, visual
detection of the marijuana in the ingested item would seem
reasonable, thus ruling out unknown consumption. Retention
time of orally ingested marijuana ranges from 1-5 days.
• Passive Inhalation: In general, routine passive exposure to
marijuana smoke will not result in a positive result for can-
nabinoids in excess of a 50 ng/mL screening cutoff.
METHODS OF ANALYSIS
The most common screening methods used to detect cannabinoids
in urine include enzyme immunoassay (EIA). Urine cannabinoid
immunoassays are usually optimized for the detection of carboxy-
THC, but also react with other cannabinoids present in the urine.
Because of this cross-reactivity, immunoassay results are expressed
in terms of “total cannabinoids” and not specically in terms of
carboxy-THC concentration as is detected by specic conrmation
methods such as gas chromatography/mass spectrometry (GC/MS)
or liquid chromatography/tandem mass spectrometry (LC/MS/MS).
Therefore, when interpreting THC concentrations, it is important to
realize that GC/MS or LC/MS/MS, which measures only carboxy-
THC, generally yields quantitative results which may represent only
10-50% of the “total cannabinoid” value as detected by immuno-
assays. While immunoassay cross-reactivity to non-cannabinoid
compounds is extremely rare, most immunoassay manufacturers
recommend that positive
results be conrmed
by alternate specic
analytical methods.
The chromatographic
methods; GC/MS and
LC/MS/MS meet this
requirement while
providing most reliable
test results.
Marijuana (THC)
Drug information
CH
3
CH
3
CH
3
CH
2
CH
2
CH
2
CH
2
H
3
C
OH
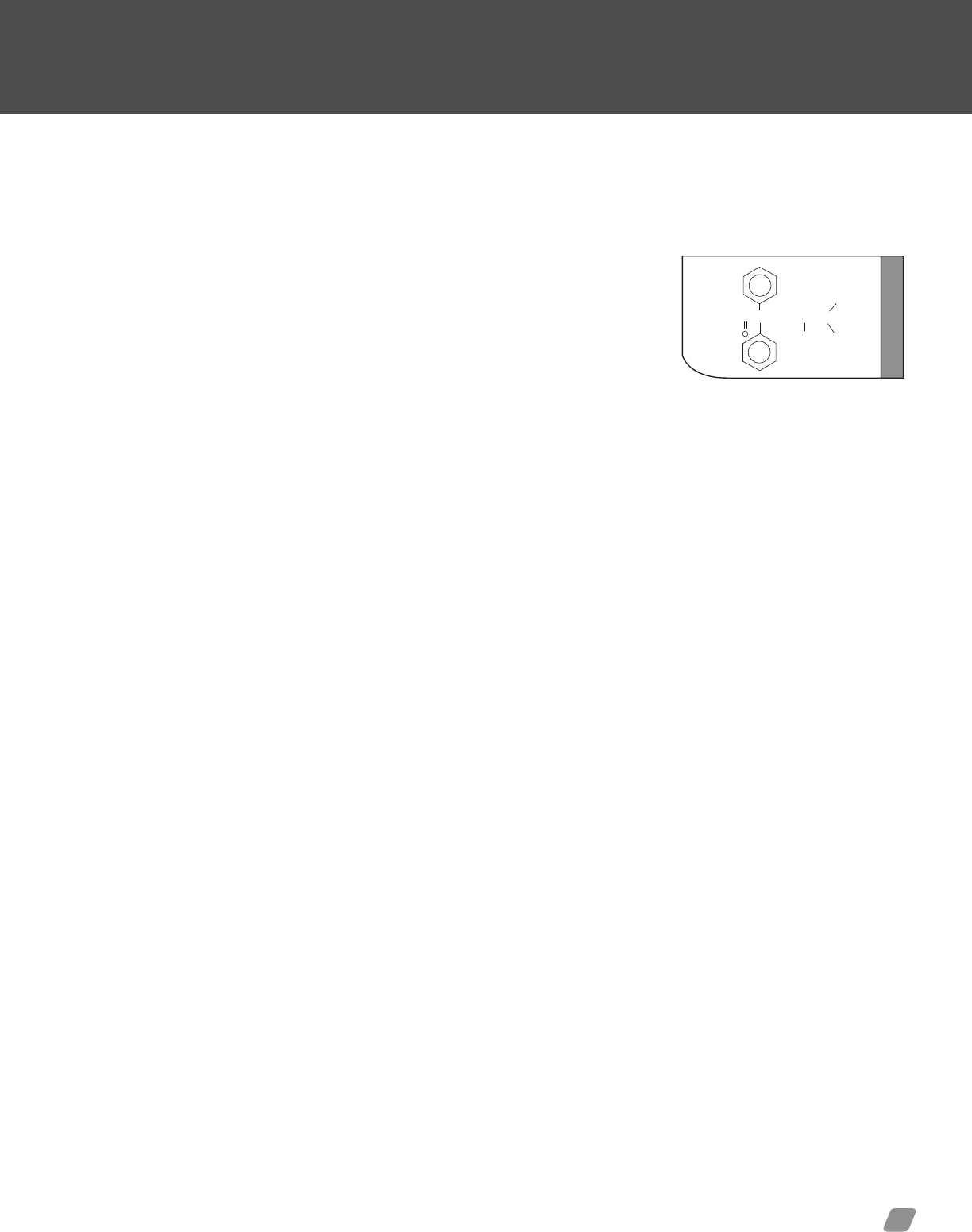
REFERENCE GUIDE // Drug information
49
CLASSIFICATION
Methadone is a narcotic analgesic which is approximately
equipotent to that of morphine. Methadone has been utilized
to treat opioid dependency and prescribed as a heroin substitute in
methadone maintenance programs since the 1960’s. Typically, daily
oral dosing with doses up to 180 mg/day, is prescribed with efcacy
measured by the absence of withdrawl symptoms. Dosing is then
gradually decreased until opiate dependency is eliminated.
METHODS OF ANALYSIS
Immunoassays, such as enzyme immunoassay (EIA) are common
methods for detecting methadone and methadone metabolite
(EDDP) in urine. Independent EIA methods are used to specically
detect methadone or methadone metabolite. Conrmation of pre-
sumptive positive urines should be performed by specic methods
such as gas chromatography/mass spectrometry (GC/MS) or liquid
chromatography/tandem mass spectrometry (LC/MS/MS).
METABOLISM
Methadone is metabolized
primarily to two pharmaco-
logically inactive metabolites,
EDDP and EMDP. Monitoring
for the presence of EDDP (methadone metabolite) is a means to
determine compliance to methadone treatment. The elimination half-
life of methadone is approximately 15-55 hours with about 5-50%
of a dose eliminated as methadone and 3-25% as EDDP. Large
individual variations in elimination do occur due to urine pH, urine
volume, dose, rate of metabolism, drug interation, etc.
Methadone
Drug information
CH3CH2C—C—CH2—CH—N
CH3
CH3
CH3
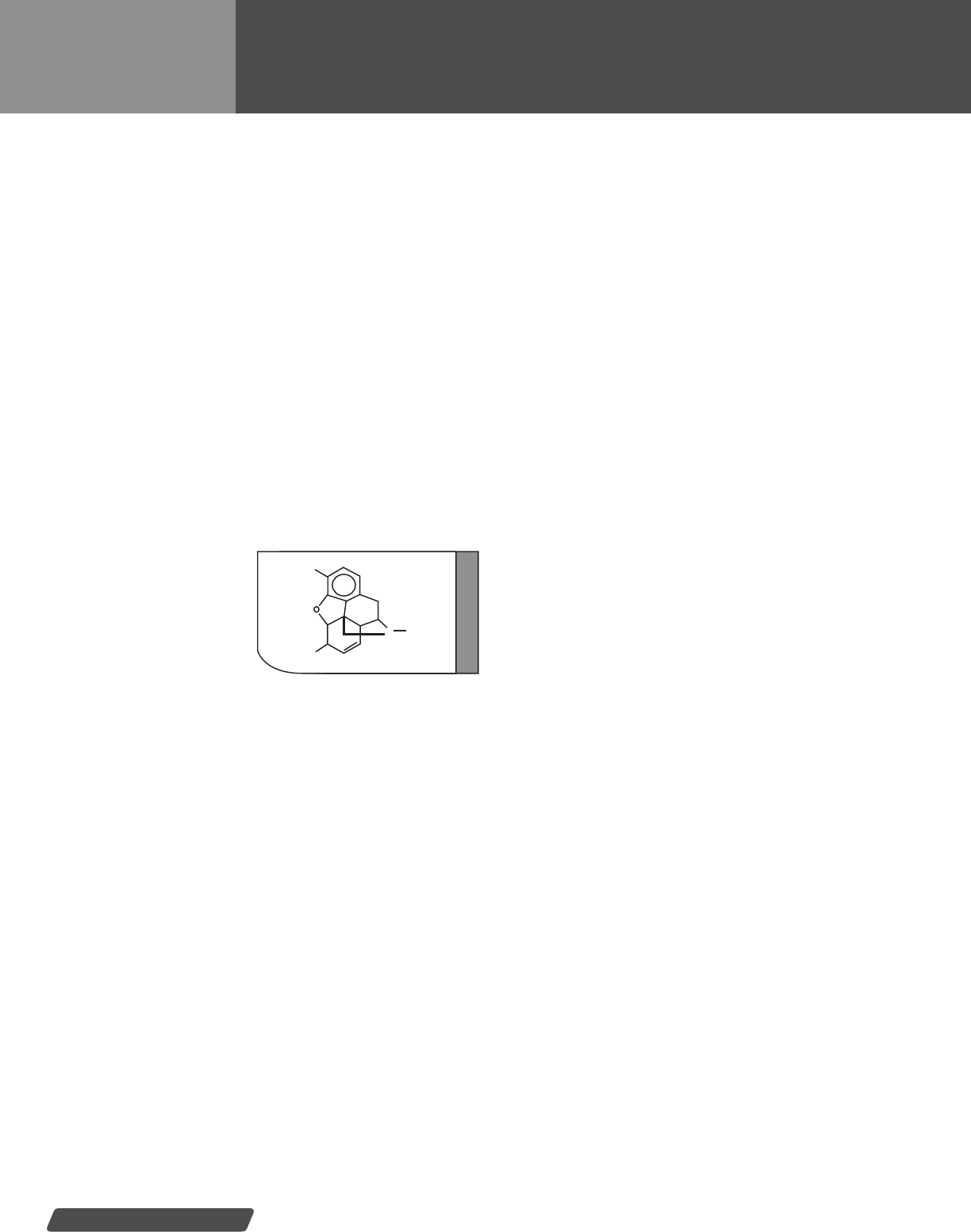
drug information
50
Opiates
Drug information
CLASSIFICATION
The term “opioid” refers to all drugs, natural or synthetic, with
morphine-like properties. Both morphine and codeine are naturally
occurring alkaloids derived from the seed pod of the opium poppy.
Semi-synthetic opiates include heroin, a diacetyl derivative of
morphine; hydromorphone, hydrocodone, and oxycodone derived by
a simple modication of the morphine molecule. Synthetic opiates
such as methadone and meperidine, mimic opiate effects but are
not prepared from the poppy. The drugs may be administered by
snorting, subcutaneous or intravenous injection, or smoking. Opioid
compounds have analgesic and antitussive properties.
METABOLISM
Morphine is rapidly
absorbed. Plasma peak
levels following an oral
dose occur after 15-60
minutes, and following IV
injection occur after 15
minutes. Extensively
metabolized by the liver,
only 2-12% is excreted as unchanged drug, while 60-80% is excret-
ed as morphine-3-glucuronide. The half-life of morphine is 1.7-4.5
hours. Heroin is rapidly metabolized (plasma half-life is 3 minutes),
rst to 6-monoacetylmorphine (6-MAM) and further to morphine.
The urinary excretion prole is similar to morphine, in that 7% is
excreted as unchanged morphine and 50-60% as glucuronides.
Trace amounts of 6-MAM, a specic metabolite of heroin, are also
excreted for approximately 6-8 hours following heroin use. Follow-
ing an oral dose, codeine is also rapidly absorbed and metabolized,
principally to codeine-6-glucuronide, with 10-15% metabolized to
morphine and norcodeine. Opiates may be detected in urine for 2-4
days following ingestion.
The interpretation of results for urines positive for opiates merit spe-
cial consideration. Since codeine is metabolized to morphine, both
substances may appear in the urine following codeine ingestion.
However, the codeine concentration is generally greater than that of
morphine. Street heroin also contains acetylcodeine, which metabo-
lizes to codeine, therefore, both codeine and morphine may be pres-
ent in the urine of some heroin users, although morphine generally
predominates. In cases of low morphine and codeine concentrations
in urine, it is not possible to determine whether codeine, morphine,
or heroin were ingested. The presence of morphine alone would
generally indicate either clinical morphine use or illicit morphine or
heroin use. A specic metabolite of heroin, 6-monoacetylmorphine,
is also at times detected and would denitely conrm illicit drug
(heroin) use. Poppy seeds, which have not been effectively washed,
contain trace amounts of codeine and morphine. When consumed
in sufcient amounts, poppy seeds may produce urines which test
positive for opiates.
ABUSE
Opioid compounds have effects on the CNS and usually on the
bowel. They produce analgesia, respiratory depression, euphoria,
mood changes, confusion, and constipation. Tolerance and depen-
dence develop with repeated use, with overdose being characterized
by coma, respiratory depression, and pinpoint pupils. Discontinuing
the drug in a dependent individual will precipitate a withdrawal syn-
drome. Heroin and morphine are the most commonly abused opioid
compounds; however codeine, propoxyphene, oxycodone, hydro-
codone, etc. are also extensively abused, as they are more readily
available. Most heroin and morphine abusers inject or “mainline”
the drugs intravenously, as this produces the most immediate and
intense effects. The heroin or morphine “rush” is the most desired
sensation which is characterized by an intense orgasmic sensation
centered in the abdomen.
METHODS OF ANALYSIS
The immunoassay methods such as enzyme immunoassay (EIA)
detect codeine and morphine in free and conjugated forms at cutoff
levels of approximately 300/2000ng/mL or less. Other opioids
detected by immunoassay at higher cutoff levels are hydrocodone,
hydromorphone, while the routine detection of oxycodone requires
a more sensitive and specic immunoassay screen. Since the
immunoassays do not distinguish between the various narcotics, a
conrmation method is required that can specically identify these
compounds. Methods commonly used include gas chromatography/
mass spectrometry (GC/MS) or liquid chromatography/tandem mass
spectrometry (LC/MS/MS). These methods determine the total
morphine and codeine content in the urine specimens. Since 90% of
these compounds are conjugated in the urine, acid or enzyme hydro-
lysis is required to convert the conjugated form into the free form.
CH
3
N
HO
HO

REFERENCE GUIDE // Drug information
51
CLASSIFICATION
Oxycontin
®
is the trade name of one of numerous Schedule II
prescription drugs that contain the opioid oxycodone as the active
ingredient. Opioids refer to a class of drugs, natural and synthetic,
with morphine-like actions. Oxycodone is reported to have equiva-
lent potency to that of morphine. Other prescription drugs that
contain oxycodone include Percodan
®
and Percocet
®
. Schedule II
drugs are those which are approved for medical use and have a high
potential for abuse and may lead to severe physical and psychologi-
cal dependence. Oxycontin
®
was rst introduced by Purdue Pharma
in 1996 as a controlled sustained release formulation for pain relief.
It was estimated that almost 6 million prescriptions for Oxycontin
®
were lled in the year 2000 and sales reached 1 billion dollars. It is
legitimately prescribed for moderate to severe chronic or long-
lasting pain. Oxycontin
®
is available in tablet forms containing 10,
20, 40, and 80 mg. A 160 mg oxycodone tablet was available which
was discontinued in May 2001. By comparison, Percocet
®
typically
contains 5 mg of oxycodone. Thus, one 160 mg tablet of Oxycontin
®
contained the equivalent amount of oxycodone as 32 Percocet
®
(5 mg) tablets.
One of the primary benets of Oxycontin
®
is that because it is
controlled release, it only needs to be taken orally every 12 hours. In
contrast, short acting oxycodone tablets like Percocet
®
require the
dosage be taken every 4-6 hours to maintain pain relief. Thus, the
controlled release formulation allows for continuous pain relief for a
substantial period of time when compared to traditional Percodan
®
or Percocet
®
tablets.
METABOLISM
A large portion of oxycodone is N-dealkylated to noroxycodone
during rst-pass metabolism. Oxymorphone, is formed by the
O-demethylation of oxycodone. The metabolism of oxycodone to
oxymorphone is catalyzed
by CYP2D6. Free and
conjugated noroxycodone,
free and conjugated oxy-
codone, and oxymorphone
are excreted in human
urine following a single
oral dose of oxycodone.
Approximately 8% to 14% of the dose is excreted as free oxycodone
over 24 hours after administration. Following a single, oral dose of
oxycodone, the mean ± SD elimination half-life is 3.51 ± 1.43 hours.
ABUSE
The attraction of Oxycontin
®
as a preferred drug of abuse over
other oxycodone containing products has several reasons. The large
amounts of oxycodone per tablet versus Percodan
®
or Percocet
®
are
one of the primary reasons, while a second factor is that Percodan
®
and Percocet
®
additionally contain 325 mg of aspirin or up to 650
mg of acetaminophen, respectively while Oxycontin
®
has neither.
Thus, the oxycodone high will not be associated with any toxic side
effects that may result from either excessive aspirin or acetamino-
phen. Oxycontin
®
tablets are to be taken whole to allow for the
controlled release of oxycodone. Abusers will destroy the controlled
release capabilities of Oxycontin
®
by either chewing the tablets prior
to swallowing, crushing the tablets and snorting the powder or they
may be crushed, dissolved in water and injected. This allows for a
rapid and large absorption of oxycodone into the blood stream pro-
ducing a powerful euphoric high. This rapid, bolus absorption is also
thought to be responsible for an apparent increase in oxycodone
related overdoses in the east. Several Oxycontin
®
street slang’s
include “OC”, “OXY”, “Oxy-Cofns”, Hillbilly Heroin, Poor-Mans
Heroin and Killers. Diversion of the prescription has also been a
problem associated with Oxycontin
®
. It has been reported that
while a single 160 mg tablet may retail for up to $14, it can fetch
up to $1 per mg on the street. Thus, a 160 mg tablet, known as a
“blue bomber”, may sell for up to $160 with its illicit sale. Interest-
ingly, Oxycontin
®
is sometimes referred to as poorman’s heroin.
We recently encountered a case in California in which a parolee
who was being prescribed Oxycontin
®
was suspected of diverting
his prescription to purchase heroin. Specic analysis of his urine
determined no detectable level of oxycodone but the primary heroin
metabolite, morphine, was present in his urine in excess of 2,000
ng/mL, thus offering scientic support to the diversion suspicion.
METHODS OF ANALYSIS
Typically oxycodone does not produce a positive response to routine
immunoassay screens for opiates, which generally target morphine
and/or codeine; therefore, Redwood Toxicology Laboratory utilizes
an enzyme immunoassay (EIA) screening method which specically
targets oxycodone at a cutoff of 300 ng/mL. Conrmation of pre-
sumptive positive urines should be performed by specic methods
such as gas chromatography/mass spectrometry (GC/MS) or liquid
chromatography/tandem mass spectrometry (LC/MS/MS).
Oxycodone
Drug information
CH
3
O
O
O
NCH
3
OH
drug information
52
Phencyclidine (PCP)
Drug information
CLASSIFICATION
Phencyclidine (l-phencyclohexylpiperidine, PCP) is prepared from
l-piperidinocyclohexane-carbonitrile in a Grignard reaction, as rst
performed in 1956 for use as an intravenous anesthetic. Pharma-
cologically PCP is classied as a dissociative anesthetic. PCP is
currently a popular drug of abuse and was once used as a veterinary
tranquilizer. A structural analog, ketamine, is currently used as a
veterinary tranquilizer. PCP is self-administered either by smoking
(drug-laced tobacco, marijuana, or parsley), by nasal insufation
and intravenous injection, or by oral ingestion.
METABOLISM
PCP is a lipophilic drug
with a large volume of
distribution. It under-
goes extensive hepatic
oxidative metabolism with
about 10-15% of a dose
excreted unchanged in
urine, and about 65% excreted as hydroxylated metabolites
and other polar metabolites. Renal excretion of PCP (pKa 8.5) is
enhanced when urine is acidic, and it is reduced when urine is
alkaline. Frequent or chronic PCP users may excrete PCP for 2-10+
days following last use. Urine concentrations may range from <0.1
mcg/mL to 340 mcg/mL.
ABUSE
Phencyclidine’s pharmacological actions are complex, since it
interacts with several neurotransmitter systems (i.e., GABAergic,
dopaminergic, cholinergic, and adrenergic). As a result, PCP has
stimulant, depressant, hallucinogenic, and analgesic properties.
Adverse effects are unpredictable and include agitation, delusions of
grandeur, anxiety, hostility, stupor, paranoia, and coma. Death has
been known to result following the ingestion of 120 mg of PCP (toxic
dose 10-20 mg).
METHODS OF ANALYSIS
The immunoassay methods (EIA) are widely used screening meth-
ods designed to specically detect phencyclidine and its inactive
metabolites. Commonly used conrmation methods include gas
chromatography/mass spectrometry (GC/MS) and liquid chromatog-
raphy/tandem mass spectrometry (LC/MS/MS). These methods offer
excellent sensitivity and specicity and are the methods of choice for
most forensic applications. False positive immunoassays have been
reported following the use of thioridazine (Mellaril), chlorpromazine
(Thorazine), dextromethorphan, or diphenhydramine (Benadryl),
therefore indicating the necessity for specic secondary
conrmation testing.
N

REFERENCE GUIDE // Drug information
53
Steroids
Drug information
CLASSIFICATION
A steroid is a terpenoid lipid characterized by a carbon skeleton with
four fused rings, generally arranged in a 6-6-6-5 fashion.
Steroids vary by the functional groups attached to these rings and
the oxidation state of the rings. Hundreds of distinct steroids are
found in plants, animals, and fungi. Many more have been produced
as synthetic drugs.
Three steroid classes found in human body belong to a subset of the
sex hormones that produce sex differences and control reproduc-
tion. They include androgens, estrogens, and progestagens.
• Androgens (androgenic-anabolic steroids) are a class of
steroids responsible for the development and maintenance
of male sexual characteristics. Androgens interact with
androgen receptors to increase muscle and bone synthesis
exhibiting their anabolic performance enhancing effect.
There are natural and synthetic anabolic steroids available.
Testosterone is a principal androgenic-anabolic steroid in the
body. In popular language the word “steroids” usually refers
to anabolic steroids.
• Estrogens are involved in female reproductive function.
• Progestagens serve as protectors of pregnancy.
• Corticosteroids include glucocorticoids and mineralocorti-
coids. Glucocorticoids regulate many aspects of metabolism
and immune function, whereas mineralocorticoids help
maintain blood volume and control renal excretion
of electrolytes.
• Cholesterol is a major source for the synthesis all other
steroid hormones in the body.
METABOLISM
In the body testosterone and its synthetic analogs are bio-trans-
formed into more polar compounds, metabolites. Enzymatically
catalyzed reduction, oxidation, hydroxylation and isomerization
are the major metabolic reactions. Consequent conjugation with
glucuronic acid or sulfate facilitates ultimate elimination of steroid
metabolites from the body with urine.
ABUSE
Androgenic-anabolic steroids (AAS) have limited medical use, but are
abused as performance enhancing drugs in sports and more recently
in some professional areas, where strong muscular appearance is
important. In the general population, especially among adolescents
and young adults, AAS are abused as a cosmetic tool helping to
improve physique.
AAS have been classied as Schedule III Controlled Substances in
the United States since 1991. Side effects include suppression of
endogenous hormone production, gynecomastia (female type breast
growth in males), acne, liver toxicity, mood swings, aggression,
infertility and masculinization in females: deepening of the voice
and male type hair growth.
T/E RATIO
Testosterone and its precursors may be endogenous (produced
in the body naturally) or exogenous (ingested as drugs or supple-
ments). The T/E ratio is used to distinguish between the two. This
ratio is a urine concentration ratio of two steroids, testosterone (T)
and its natural isomer, epitestosterone (E). The normal average ratio
is approximately 1, with individual variation on both sides, either
higher or lower.
Ingestion of exogenous testosterone or its precursors suppresses
internal steroid production in the body. Both endogenous T and E
would be suppressed. However, total testosterone concentration
in urine will rise above normal due to ingested drug. Low E and
high T cause the T/E ratio to rise above 6 (cutoff), indicating
testosterone abuse.
METHODS OF ANALYSIS
RTL utilizes the most sophisticated, sensitive, and specic equip-
ment and technology available. RTL performs a fast, efcient and
sensitive GC/MS steroid screen, capable of detecting 85 endog-
enous and exogenous compounds providing information about
naturally occurring and synthetic steroids and metabolites. The RTL
Steroid Test includes specic GC/MS conrmation methods for each
individual drug.
Accurate quantication is performed for nandrolone and testos-
terone with cut-off levels of 2 ng/mL (nandrolone metabolite) and
testosterone to epitestosteroine ratio above 6 (T/E > 6).

drug information
54
Stimulants
Drug information
CLASSIFICATION
Stimulants are psychoactive drugs that induce temporary improve-
ments in mental and/or physical function. The effects of stimulants
include enhanced alertness, wakefulness, endurance, productivity
and motivation; increased arousal, locomotion, heart rate/blood
pressure; and a perception of a diminished requirement for sleep.
Symptoms of excessive stimulation of the central nervous system
(CNS) include restlessness, difculty sleeping, tremor, headaches
and even psychotic episodes. Loss of appetite (anorexia) and weight
loss also occur with CNS.
Most stimulants are classied as Controlled Substances (CS) in the
United States. Designer drugs like MDMA (ecstasy), which have
no medical use, are classied as Schedule I CS; the others belong
to Schedule II – IV substances. There are several categories of
stimulants possessing different chemical structures and employing
different pharmacological mechanisms:
• Amphetamine, Methamphetamine, their precursors and
structurally related designer drugs represent a broad range
of classic stimulant agents.
• Cocaine is the most potent of naturally-occurring stimulants.
It is commonly abused and a highly
addictive drug.
• Piperazines, such as benzylpiperazine and triuoromethylpi-
perazine, possess stimulant and hallucinogenic activity. They
became especially popular worldwide as party drugs in the
recent years.
• Eugeroics, like adrtanil and modanil, are prescribed for
the treatment of narcolepsy (excessive daytime sleepiness).
They were abused as “wakefulness promoting agents” and
as CNS stimulants in sports.
• Methylxanthines, such as caffeine, are found in tea, coffee
and “energy” drinks. They are also potent stimulants. How-
ever, their use is widely accepted in the society.
• Nicotine in tobacco products is also a stimulant, which is not
currently banned.
• Other Stimulants—this is a broad group of Controlled Sub-
stances with different chemical structures and common CNS
pharmacological effects. Many of them, like fenuramine
and phentermine, were originally designed as weight loss
drugs. They are no longer used due to severe side effects.
METABOLISM
Most stimulants are excreted in urine unchanged with only minor
metabolites. Numerous precursor drugs metabolize extensively
into active amphetamine and methamphetamine. Cocaine is rapidly
inactivated in the body and excreted with urine fully metabolized.
ABUSE
Stimulants may cause extreme psychological dependence. As drugs
of abuse, stimulants are frequently taken to produce a sense of
exhilaration, enhance self esteem, improve mental performance,
increase activity, produce prolonged wakefulness, and to “get high”.
Stimulants are also popular as appetite suppressants. Ability to
improve short-term physical performance made stimulants one of
the most abused drug classes in sports.
METHODS OF ANALYSIS
Immunoassay screens with gas chromatography/mass spectrometry
(GC/MS) or liquid chromatography/tandem mass spectrometry
(LC/MS/MS) for conrmation is a classic approach for amphet-
amines and cocaine analysis in urine. RTL’s comprehensive stimu-
lant test for athletic doping control employs gas chromatography/
mass spectrometry (GC/MS).

REFERENCE GUIDE // Drug information
55
Synthetic Cannabinoids
Drug information
CLASSIFICATION
Synthetic Cannabinoids are chemicals that act as cannabinoid
receptor agonists. Chemically they are not similar to cannabinoids
but the term “Synthetic Cannabinoids” or “Cannabinomimetics”
is widely used to refer to them as they’re cannabinoid-like in
their activity.
The synthetic cannabinoid receptor agonists fall into seven major
structural groups:
• Naphthoylindoles (e.g. JWH-018, JWH-073)
• Naphthylmethylindoles* (JWH-185, JWH-199)
• Naphthoylpyrroles* (JWH-369, JWH-370)
• Naphthylmethylindenes* (JWH-176)
• Phenylacetylindoles (JWH-250)
• Cyclohexylphenols (e.g. CP 47,497 and
homologues of CP 47,497)
• Classical cannabinoids (e.g. HU-210)
*Compounds in these groups have not been detected in herbal blends so far.
METABOLISM
Little is known about the detailed pharmacology and toxicology of
the synthetic cannabinoids and few formal human studies have
been published.
Synthetic Cannabinoids metabolize extensively in humans via oxida-
tion and glucuronide conjugation. Following a single low dose, the
hydroxylated synthetic cannabinoids and the carboxylated synthetic
cannabinoids metabolites can be detected up to 72 hours in urine.
Very little parent drug excreted in human urine has been reported.
In case of chronic use the detection window could be longer.
Presence of parent drug in saliva conrms ingestion; average
detection window up to 24-48 hours.
It is possible that, apart from high potency, some other synthetic
cannabinoids could have particularly long half-lives, potentially
leading to a prolonged psychoactive effect. In addition, there is
considerable inter-and intra-batch variability in smoking mixtures,
both in terms of substances present and their quantity. Thus, there
is a higher potential for overdose than with cannabis.
ABUSE
Initially, JWH-018 and JWH-073 were the two most common
synthetic cannabinoid chemicals found in a variety of herbal smok-
ing blends. Others like AM-1248, AKB-48, UR-144, and XLR-11
have started appearing in newer synthetic cannabinoid products
and preparations. Reportedly offering a high 4 times stronger than
marijuana, these compounds are commonly associated with herbal
smoke and incense products sold under names such as K2, K3
Legal, Spice, Syn, Haze, Cloud Nine, Serenity and many others.
Synthetic cannabinoid chemicals are often laced in the herbal smok-
ing products that are readily available via the Internet and in many
“head-shops” around the country.
Users looking for a “high” often turn to these herbal smoking or
incense products because they do not show up on a standard urine
drug test. Users smoke the product by wrapping joints, smoking it in
pipes, or inhaling fumes via vaporizers. Users also report that herbal
blends or pure chemical concoctions can be ingested with an infu-
sion or solvent process; purportedly allowing them to manage the
potency and dose of the active ingredient(s).
Users indicate the high comes on slow at rst, then with surprising
potency. There have been many reports about the adverse effects
including agitation, rapid heart rate, confusion, dizziness and nausea.
According to the American Association of Poison Control Centers,
the number of human exposure calls relating to synthetic canna-
binoids was 6,968 and decreased over the following years. As of
August 2014, the total volume of calls regarding synthetic cannabi-
noid exposures is still over 2,000 showing that the true extent of the
public health threat is still prevalent.
Long-term effects from these research chemicals are unknown.
In July 2012, the DEA banned synthetic cannabinoids based on
their structural classication, explicitly naming 15 chemicals, citing
numerous calls to poison control centers around the nation. In May
2013, the DEA placed a temporary ban on three additional synthetic
cannabinoid substances. However, newer generation compounds
continually emerge—making it more vital than ever to target syn-
thetic marijuana.
METHODS OF ANALYSIS
Immunoassay screens are now available for synthetic cannabinoid
testing. RTL’s test utilizes the most sophisticated, sensitive and
specic equipment and technology available, liquid chromatography/
tandem mass spectrometry (LC/MS/MS) to conrm presumptive
positive specimens for synthetic cannabinoid metabolites in urine.
The method relies on monitoring multiple metabolites for each drug.
RTL’s test methodology provides the most denitive synthetic can-
nabinoid biomarker test results.
For oral uid testing, LC/MS/MS is utilized to conrm parent syn-
thetic cannabinoid drugs.

drug information
56
Urine Creatinine
Drug information
CLASSIFICATION
Creatinine is a metabolic by-product of muscle metabolism, and
normally appears in urine in relatively constant quantities over a 24
hour period with “normal” liquid intake. Therefore, urine creatinine
can be used as an indicator of urine water content (dilution) or as a
marker identifying a specimen as urine. Greater than normal intake
of water will increase the urine water content (lowering the creati-
nine level) consequently diluting any drug which may be present in
urine. Conversely, a limited intake of water can lead to an abnor-
mally concentrated urine specimen (as occurs with dehydration)
resulting in elevated creatinine levels.
INTERPRETATION OF RESULTS
Creatinine Conc.: Interpretation:
<20 mg/dL Dilute urine specimen: Most likely due to
increased water or liquid intake. Can be a result
of short-term water loading (ushing) in an
attempt to dilute any drug below testing cutoff
concentrations.
<2.0 mg/dL Abnormally dilute: Specimen showing an
excessively low creatinine value. May be an
indication that the specimen is not consistent
with normal human urine.
NOTE: The above values are based on the critical points
that the Federal Department of Health and Human Services,
Substance Abuse Mental Health Services Administration (SAMHSA)
has set as decision points for interpreting dilute or substituted
urine specimens.
The above interpretations are general guidelines only. Redwood
Toxicology Laboratory recommends consulting with a certied
toxicologist regarding proper interpretation prior to taking adminis-
trative action based solely on the creatinine concentrations. Other
physiological conditions may account for low creatinine concentra-
tions such as diabetes or use of prescription diuretics.

REFERENCE GUIDE // Drug information
57

contact information
58
Get in touch with us.
SALES AND
GENERAL INQUIRIES
RTL offers toll-free sales and customer support services during regular business hours.
RTL hours are 7:30 AM to 4:00 PM PST.
Phone: Laboratory Services – 800.255.2159, press option 1 or 707.577.7959
Screening Devices – 877.444.0049 or 707.571.1157
Fax: 707-577-8102
E-mail: sales@redwoodtoxicology.com
Address: P.O. Box 5680, Santa Rosa, CA 95402 or
3650 Westwind Blvd., Santa Rosa, CA 95403
TOXICOLOGY
SUPPORT SERVICES
Trained customer support representatives are available to assist you in a prompt and
courteous manner. We’ll get you up and running on specimen collection, results
interpretation and technical inquiries.
Phone: 800.255.2159, press option 5 or 707.577.7959
Fax: 707.577.0365
E-mail: clientservices@redwoodtoxicology.com
INFORMATION
TECHNOLOGY
IT is available during regular business hours to answer client inquiries about internet
(www.RedwoodToxicology.com) and statistical reporting. Contact IT to request your
ToxAccess
™
username and password for convenient results retrieval.
Phone: 800.255.2159, ext. 34311 or 707.577.7959
Fax: 707.577.0 471
E-mail: helpdesk@redwoodtoxicology.com
TESTING SUPPLIES
To request labels, bottles and shipping materials, contact our Supplies Department.
Lab supply re-ordering is available to existing clients with an account number.
Phone: 800.255.2159, press option 4 or 707.577.7959
E-mail: supplies@redwoodtoxicology.com
Web: https://www.redwoodtoxicology.com/resources/supply_form
SCHEDULING A
SPECIMEN PICKUP
MORE THAN 3 PICKUPS A WEEK—RTL will arrange for daily FedEx pickups.
A FedEx courier will stop at your location daily (M-F) and pick up your specimen boxes.
Please do not arrange pickups directly as this may result in your agency being billed for
these services.
LESS THAN 3 PICKUPS A WEEK—To schedule a pickup, change a previously
scheduled pickup or cancel/suspend a pickup, please call FedEx directly at 1-800-GoFedEx
(1-800-463-3339). For assistance nding a drop-off location visit fedex.com.
To contact Redwood Toxicology Laboratory (RTL), please select from the
departments listed below. All requests made during non-business hours
will be responded to as early as possible the next business day.

59
CONFIDENCE IN TESTING
Redwood Toxicology Laboratory, Inc. (RTL) is the preferred choice
in drug testing providers. RTL provides accurate laboratory testing
services for drugs of abuse in urine and oral uid, synthetic can-
nabinoids, designer stimulants, EtG/EtS, steroids, GHB, fentanyl
and more. We also sell instant, on-site drug and alcohol screening
devices. Our reputation for experience, accuracy and excellence in
customer service speaks for itself.
CONTACT US TODAY!
Address: 3650 Westwind Blvd.,
Santa Rosa, CA 95403
Laboratory Services: (800) 255-2159
Screening Devices: (877) 444-0049
Fax: (707) 577-0365
Email: sales@redwoodtoxicology.com
Website: www.redwoodtoxicology.com
11 000 3034 REV10
© 2014 Alere. All rights reserved. Reditest, ToxAccess and the Redwood
Toxicology Laboratory logo are trademarks of the Alere group of companies.
